Surgical Treatment of Keloid Lesions: Help or Harm?
Results of an online survey and a discussion of alternative therapies for early-stage keloid lesions
Michael H. Tirgan, MD
ABSTRACT
Background: Many patients undergo surgical removal of their keloids. Although there are several reports about the utility and efficacy of surgery for the treatment of keloid lesions, few studies have reported on how patients perceive the outcomes of this intervention.
Objective: To assess patients’ perceptions of the efficacy and ill effects of surgery for the treatment of keloid lesions.
Material and Methods: An online survey was launched in November 2011 asking participants to provide answers to questions about their keloids. Patients were asked about their perceptions of the efficacy and long-term results of common treatment modalities. Descriptive statistics are provided.
Results: As of December 4, 2021, 1873 individuals participated in this survey, 567 of whom reported previous surgical removal of their keloids. Of the 567 patients, 548 assessed the benefit of this intervention: 27 (4.93%) reported that surgery cured their keloids, 91 (16.61%) benefited from surgery, 122 (22.26%) showed no improvement, and 308 (56.20%) showed worsening of keloids following surgery.
Conclusions: Results show that 56.20% of patients reported worsening of their keloids after surgical intervention. Although recurrence of keloids is widely acknowledged and reported, the occurrence and rate of worsening of keloids after surgery are not routinely reported in the surgical literature. This study represents the first step in developing a patient-reported measure of treatment success from surgery. Alternative therapies for early-stage keloid lesions were discussed.
INTRODUCTION
Surgical removal of keloid lesions is widely practiced by many dermatologists and plastic surgeons. However, because this treatment approach has been associated with recurrence rates of up to 100% [1], various postoperative adjuvant treatments have been used to reduce the rate of recurrence after surgery.
Gold et al. (2020) advocated adjuvant radiation therapy and concluded that keloidectomy followed by radiation therapy provided satisfactory recurrence rates, but they disclosed that their manuscript was funded by the maker of dermatological radiation therapy equipment [2]. Although the authors provided a thorough review of the literature on adjuvant radiation therapy and commented on the risk of recurrence of keloids, they did not mention the risk of worsening of keloids after surgery.
Ogawa et al. (2020) recommended a multimodal treatment strategy that included postoperative radiation therapy [3]. The authors discussed the risk of keloid recurrence but, similar to Gold et al., did not mention worsening of keloid as a risk of their treatment approach.
Lemperle et al. (2020) reported on their own experience and outcomes of surgical removal of keloids in 452 patients in Africa [4]. Although the authors presented several cases of massive keloids and mentioned that some of their cases had had prior surgery, they likewise did not conclude that prior surgery was the cause for worsening keloids in any of their patients.
In their thorough review of keloid disorder (KD), Limandjaja et al. (2020) mentioned worse outcomes after surgical resection [5]. Although worsening keloid is mentioned in their publication, it appears only by way of providing references to Robles and Berg 2007 [6], Butler et al. 2008b [7], Balci et al. 2009 [8], and Shih et al. 2010 [9]. The authors did not publish or discuss any of their own data on the rate of worsening of keloids after surgery among their own patients.
Robles and Berg (2007) stated, “excision often results in a longer scar than the original keloid, and recurrence in this new area of trauma can lead to a larger keloid” [6]. This statement about the worsening of keloids after surgery, however, was attributed to Poochareon and Berman [10]. Likewise, the statement by Poochareon and Berman (2003) that “keloids are likely to worsen or recur after surgical excision” was attributed to Shaffer et al. (2002) and, much like other authors, did not provide any primary research data on the rate of worsening of keloids after surgery among their own patients.
Shaffer et al. (2002) conducted a retrospective review of several studies on the treatment of keloids and stated that their “objective was to examine the scientific quality of the literature on therapy for keloidal scars” [11]. The authors did not present any of their own clinical data. In the same publication, the authors stated, “there are many problems with the study designs of existing keloidal scar research including lack of consistent disease definitions and outcome measures, inadequate follow-up, and inconsistent therapeutic interventions.” Unfortunately, the same issues that were noted by Shaffer et al. in 2002 remain unchanged in 2021. Although the manuscript of Shaffer et al. contains 165 references, the term worsening of the keloids in the manuscript appears without a proper reference.
Butler et al. (2008) mentioned recurrence of keloids after surgical resection but not worsening of the keloids after surgery [7]. Similarly, Balci (2009) did not address the worsening of the keloids and was more focused on the quality of life of keloid patients [8].
Shih et al. (2010) mentioned worsening of keloid after surgery [9], but only by citing Robles and Berg [6]. The authors offered no reference to the worsening of keloids after surgery and did not provide primary research data.
Cognizant of recurrence of keloids after surgery, many authors have suggested incorporation of various adjuvant therapy methods, such as corticosteroid injections [12], radiation therapy [3], and pressure devices [13], to diminish the rate of postoperative recurrence. However, despite these efforts, postoperative recurrence remains a real challenge and a cause of disappointment for both patient and physician. Bennet et al. (2017) conducted a retrospective review of their experience with postoperative radiation therapy and reported a recurrence rate of 74% among 31 patients who were followed for at least 1 year [14]. The authors did not mention the worsening of keloids in their report.
Only Escarmant et al. (1993) properly referenced the worsening of keloids [15]. The authors reported their experience of 544 patients with 783 keloids who were treated in Martinique with surgery and adjuvant interstitial radiation therapy. The authors were able to obtain in-person follow-up of 361 patients with 570 treated keloids. Fifty percent of their patients were younger than 20 years of age; 51.4% of their patients had earlobe keloids. The authors reported that 450 keloids (81.1%) had improved, 52 keloids (9.4%) had worsened, and 53 keloids (9.5%) were stable.
Among the recurring keloids (118), 60 (50.8%) had improved, 38 (32.2%) had worsened, and 20 (17.0%) were stable. For non-recurring keloids (437), 390 scars (89.2%) had improved, 14 scars (3.2%) had worsened, and 33 scars (7.6%) were stable.
In this study, the author attempts to place a spotlight on the worsening of keloids after surgery, an important and largely neglected topic. An institutional review board (IRB)-approved online keloid survey was launched by the author in November 2011 to inquire about different aspects of KD, including the efficacy and potential side effects of various treatment modalities. The present study reports on the self-assessments of patients who completed the survey.
MATERIALS AND METHODS
A comprehensive questionnaire was developed to survey a large cohort of consecutive unselected patients with KD. The study was initially approved in November 2011 by the IRB of St. Luke’s Roosevelt Hospital in New York. The study was subsequently transferred to Western IRB.
The survey links were placed in various websites. Participants would come across the link on the internet, and if they were interested in participating in the study, they would have to access the study questionnaire by visiting the study website, www.KeloidSurvey.com. After downloading and reviewing the study consent form, adult participants were asked to provide informed consent electronically. Parents were able to consent and complete the survey on behalf of their underage children.
The survey posed numerous questions to the participants, assessing a patient’s age, ethnic background, family history, extent and distribution pattern of the keloid lesions, prior treatments and response rates, and participants’ perception of benefit from their treatments. However, the survey did not collect data regarding the nature or timing of postoperative adjuvant treatments, location of the excised keloids, or any other specific information about surgical methods.
Participants’ access to the survey tool was limited to one access per computer IP address. Participants were allowed to skip a question if it did not apply to them, or they did not wish to answer. The study dataset was accessed on December 4, 2021. Descriptive statistics and the perceptions of the survey participants about the benefit they might have gained from keloid removal surgery are presented in the following section.
RESULTS
The study was opened for accrual on November 14, 2011. As of December 4, 2021, 1873 individuals had participated in this survey; 567 adult participants (156 men [27.9%]) and 403 women [72.1%]) indicated that they had previously undergone surgical treatment of their keloids.
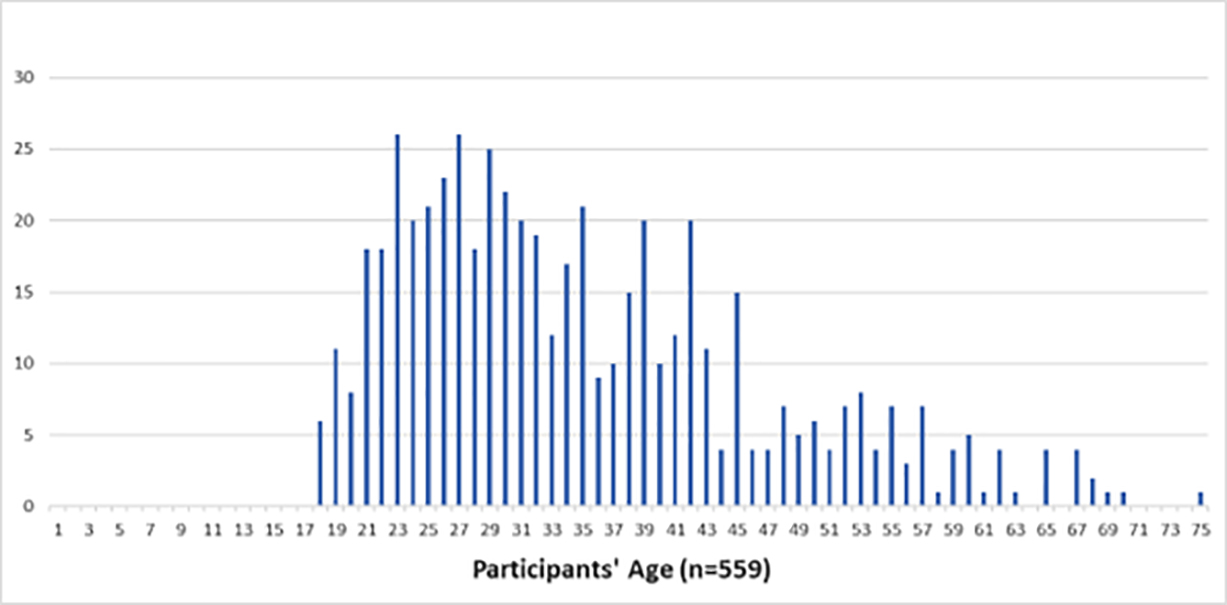
FIGURE 1: Participants’ age at the time they took the survey.
Age of onset/clinical presentation of KD
Participants were also asked to provide their age at the time they developed their first keloid lesion; 559 participants provided an answer. The peak age of onset among all participants was 16 years; 481 participants (89.3%) had developed their very first keloid between the ages of 5 and 25 years.
The majority of participants (359, or 66.4%) were between the ages of 1 and 17 years when they developed their first keloid, making KD a predominantly pediatric illness.
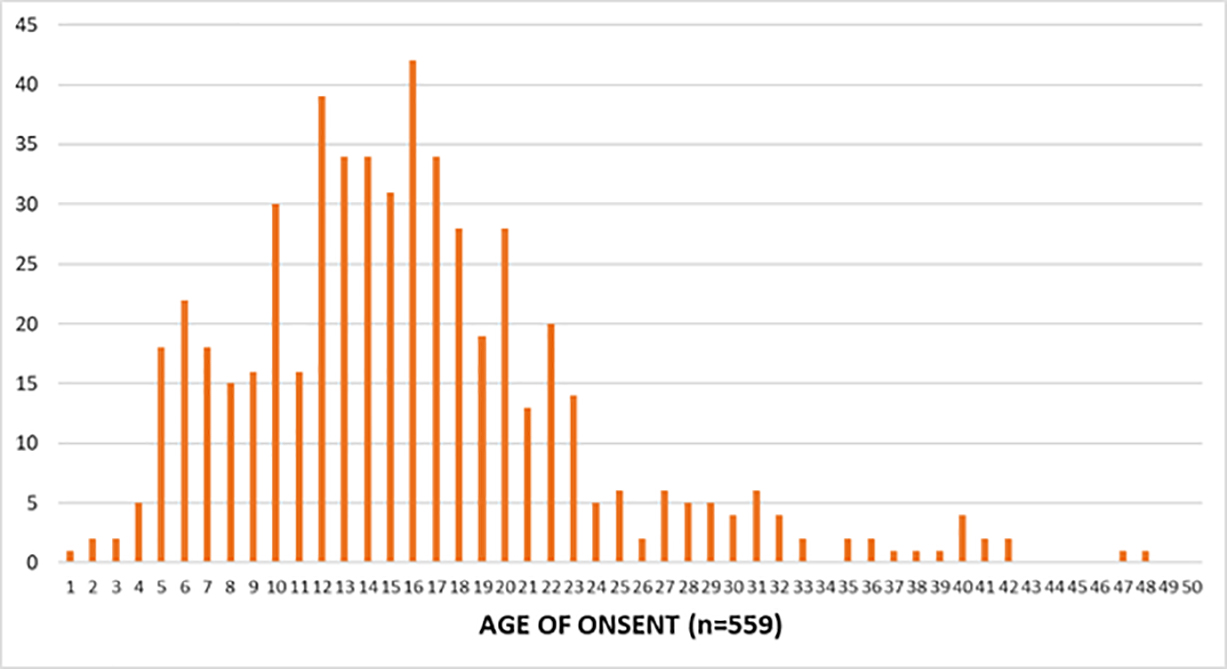
FIGURE 2: Age of onset/clinical presentation of KD among the study population.
Country of Birth
A total of 560 participants provided information about their country of birth. The majority of the participants (62.14%) were born in the United States, 3.21% in India, 2.68% in the United Kingdom, and 2.50% in Canada. Figure 3 depicts the country of birth of all study participants.
Although this distribution pattern correctly represents the country of birth of those who participated in this study, it is by no means a true reflection of the epidemiology of KD. Lack of access to the internet in many regions worldwide, as well as the fact that the survey has been available only in English, limits the availability of the survey to many keloid patients.
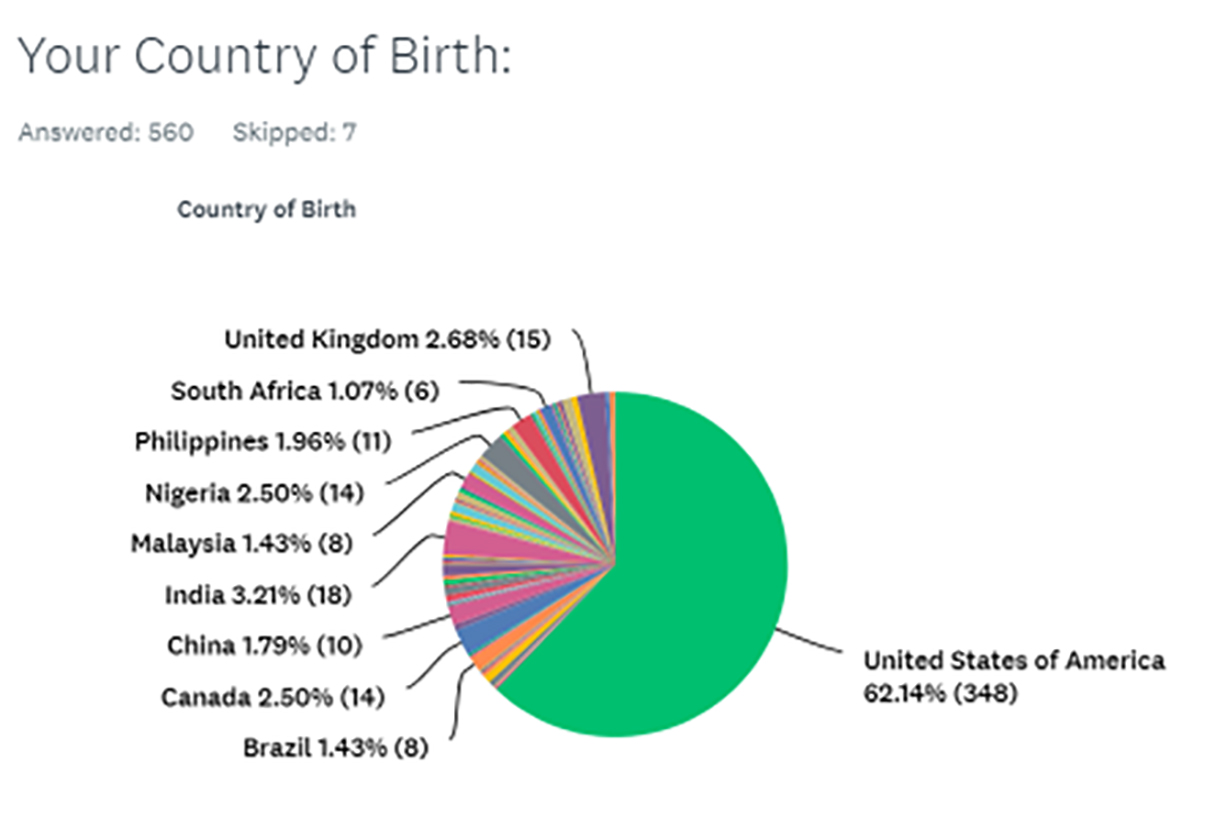
FIGURE 3: Country of birth of the study participants (n = 560).
Ethnicity
Participants were asked to provide their ethnic background, and 563 provided this information. Figure 4 shows percentages for the ethnicities of the respondents. Although this information is a correct representation of those who participated in this study, it is not a true reflection of the ethnic epidemiology of KD.
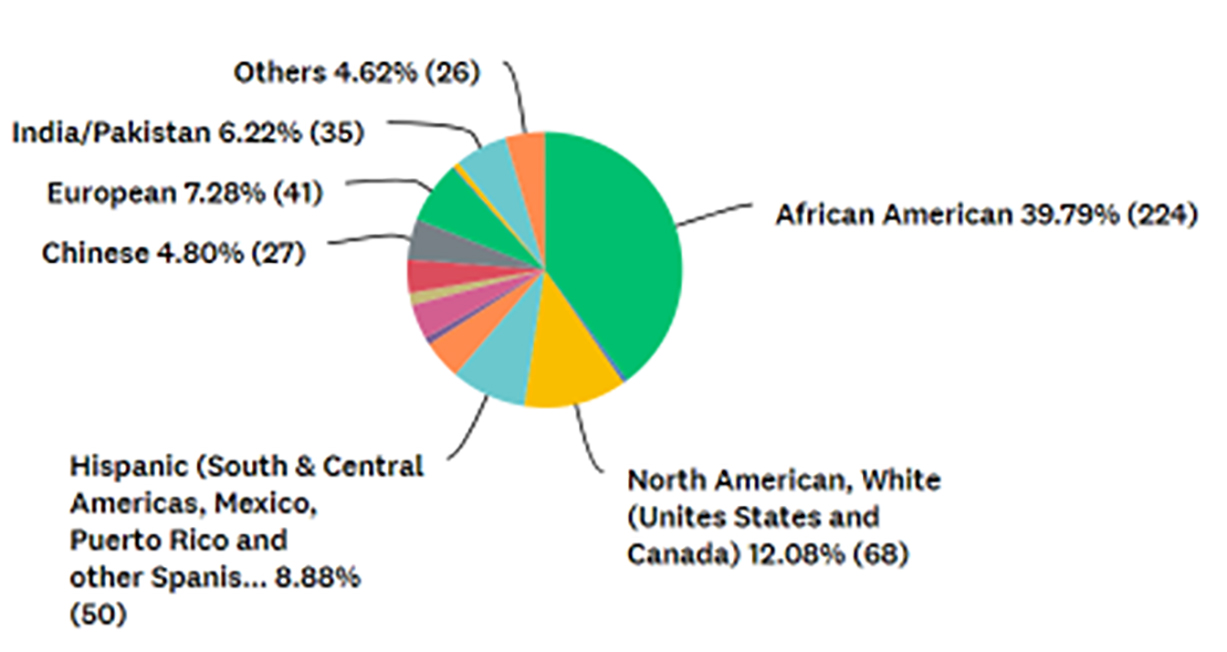
FIGURE 4: Participants’ ethnic backgrounds (n = 563).
Pattern of Distribution of Keloid Lesions
Participants were asked to provide detailed information about the distribution of the keloid lesions throughout their skin. A total of 546 participants provided this information. Participants who had multiple keloids in different parts of their skin were asked to indicate all sites of disease involvement. The upper arm, ear, lower chest, and pelvis were the most frequently involved area of the skin among the study participants.
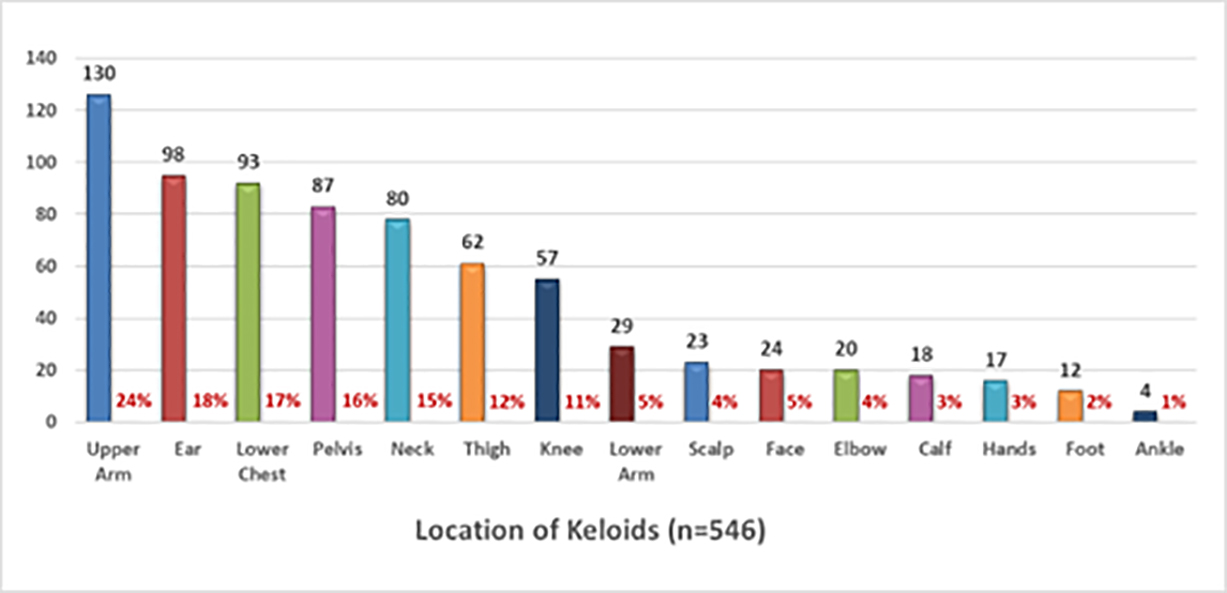
FIGURE 5: Pattern of distribution keloid lesions among study participants.
Morphology of Keloid Lesions
Participants were asked to describe the shape and appearance of their keloid lesion. To facilitate this, a reference image guide was provided online. A total of 560 participants provided this information. Participants who had multiple keloids with different morphologies were asked to describe each type of keloids.
Nodular morphology was reported in 61% of participants followed by flat keloids in 47% and linear keloids in 46% of the participants. Thirty-four percent of patients considered their keloids to be massive, with keloid lesions occupying large areas of their skin.
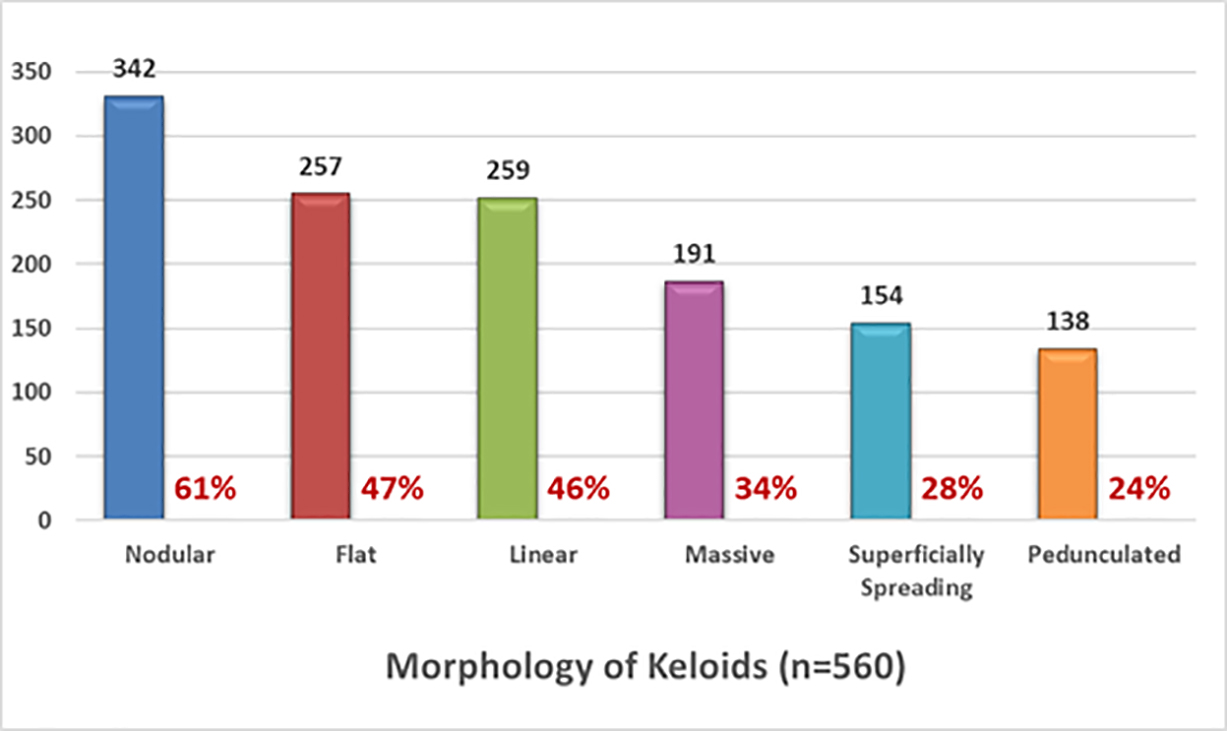
FIGURE 6: Morphology of keloid lesions among study participants.
Triggering Factors
Participants were asked to provide information about the factors that triggered the formation of their keloids; 535 participants answered this question. Figure 7 shows the frequency of various triggering factors within this population.
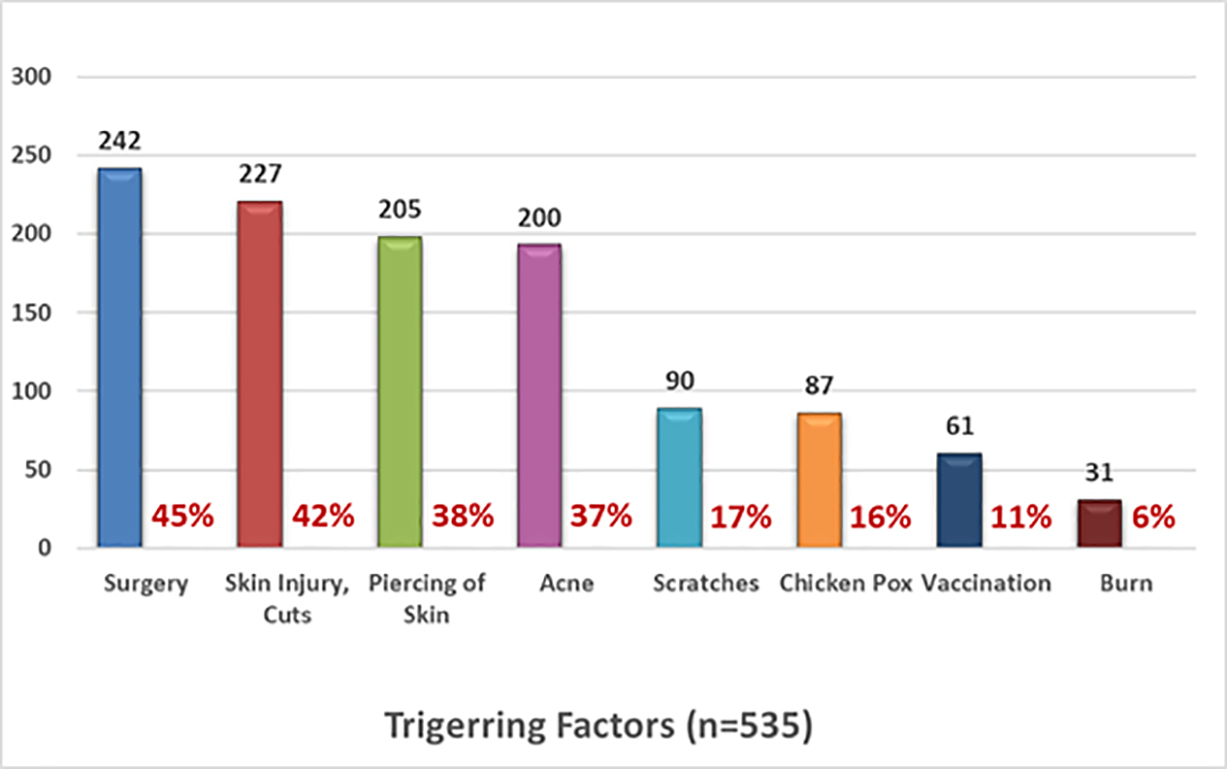
FIGURE 7: Triggering factors reported by participants.
Number of Keloids
The participants were asked to provide information regarding the number of keloid lesions that they had at the time of taking the survey. A total of 556 participants provided this information. Figure 8 depicts the distribution pattern of the number of keloid lesions.
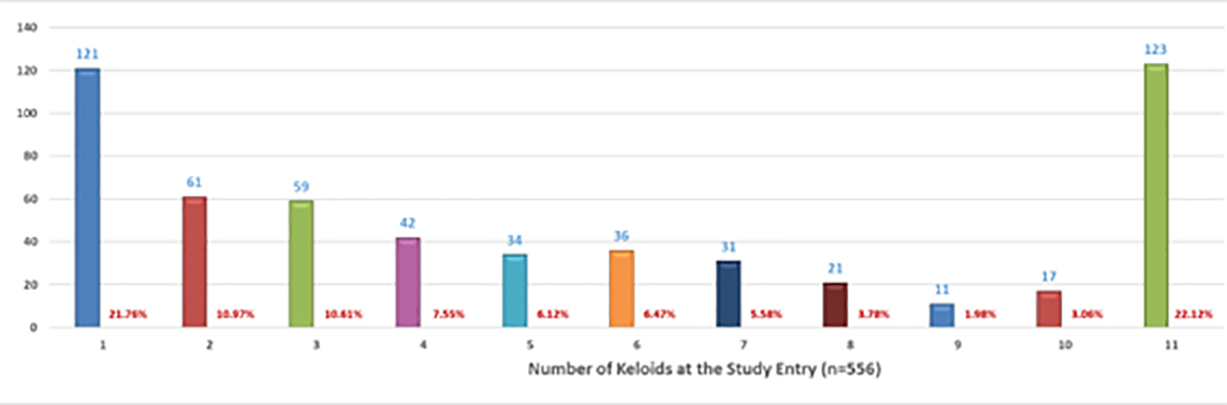
FIGURE 8: Distribution pattern of the number of keloid lesions in each participant.
Efficacy of Surgery
The efficacy of keloid removal surgery was assessed by asking participants to provide only one answer to each of two sets of multiple-choice questions that would explore observed efficacy and overall assessment of benefit from surgery. Figures 9 and 10 depict the responses by the 554 participants who answered questions about response to treatment and benefit of surgery, respectively.
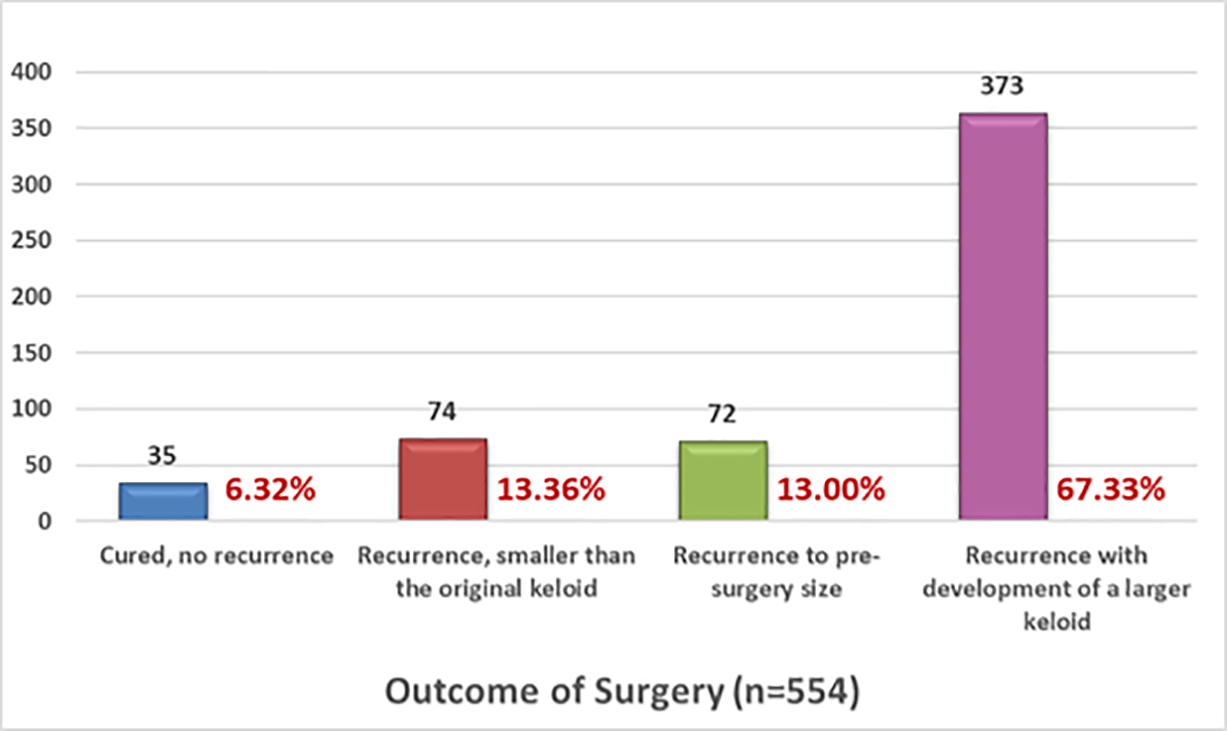
FIGURE 9: Recurrence rates after surgery, self-reported by patients.
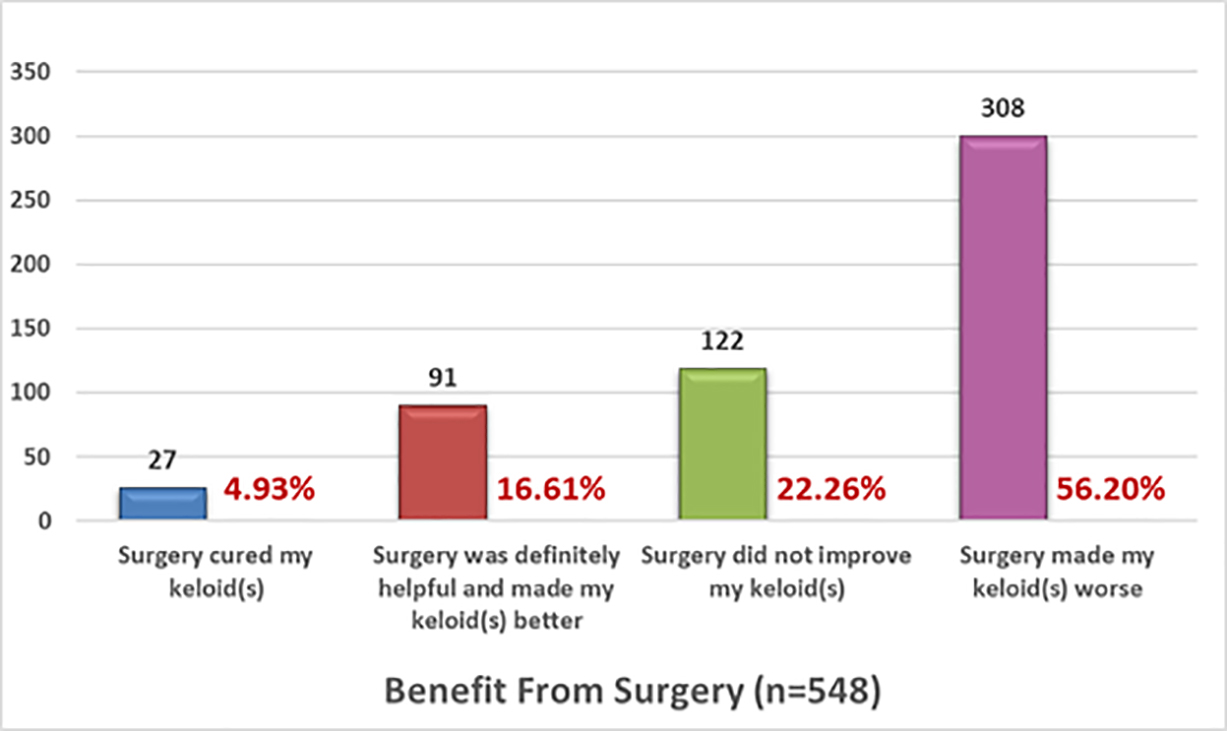
FIGURE 10: Benefit received from surgery, self-reported by patients.
To further refine the survey results, data were analyzed to identify patients who reported having a solitary keloid lesion. A total of 306 participants reported that they only had one keloid lesion, and 121 participants responded that they underwent surgery for their solitary keloids. A total of 117 participants responded to the outcome questions, among whom 115 participants provided detailed information regarding the distribution of the keloid lesions throughout their skin. Among this subpopulation, the most frequently affected areas were the upper chest (22%), ear (20%), earlobe (20%), shoulder (8%), neck (8%), and lower chest (4%). Figure 11 depicts the outcomes of the 117 participants who answered questions regarding the treatment response to surgery.
As shown in Figure 11, the reported surgical outcomes among these patients were very similar to those of the whole group, wherein 65% of the patients reported the worsening of their keloids after surgery. The survey did not specifically collect information regarding prior treatments, the type or timing of adjuvant treatments, or other factors that might have affected the treatment outcomes.
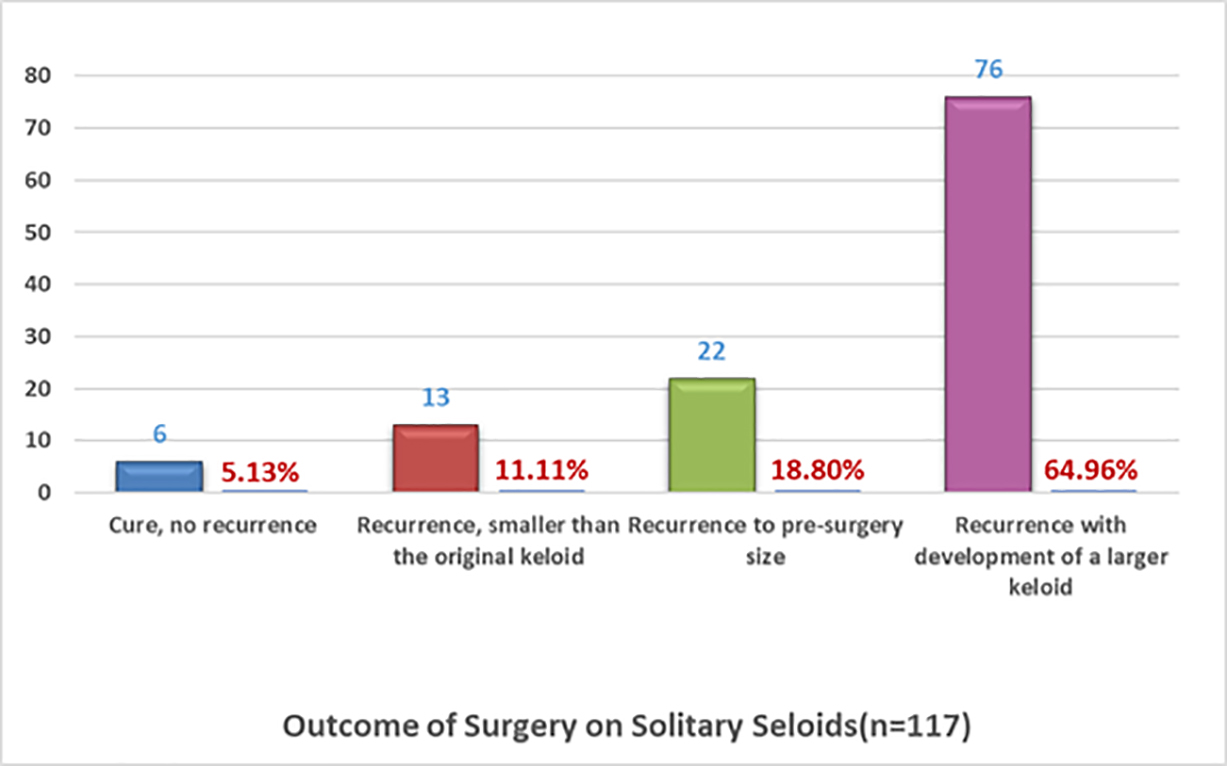
FIGURE 11: Recurrence rates after solitary keloid surgery as was self-reported by patients.
Twenty-six participants also reported having undergone adjuvant radiation therapy along with having solitary keloids; hence, the survey was able to assess the effect of adjuvant radiation therapy in this small sub-population. However, the survey did not collect any details regarding radiation therapy. It was assumed that radiation therapy was delivered as an adjuvant therapy following the excision of solitary keloids. A total of 18 participants provided information about the location of their keloids, 10 had ear or earlobe keloids, 4 had shoulder keloids, 3 had lower chest keloids, 1 had a neck keloid, and 1 had an upper chest keloid.
As shown in Figure 12, the reported outcomes of surgery followed by radiation therapy among these participants were very similar to those of the whole group. Nearly 81% of the participants reported worsening of their keloids following surgery. The survey did not collect information regarding prior treatments, including prior surgery, and the timing of surgery or radiation therapy. The outcomes reported by this small group of participants were very similar to that of the rest of the participants. The outcomes of these participants may not truly reflect the outcomes of surgery followed by radiation therapy in prospectively planned studies.
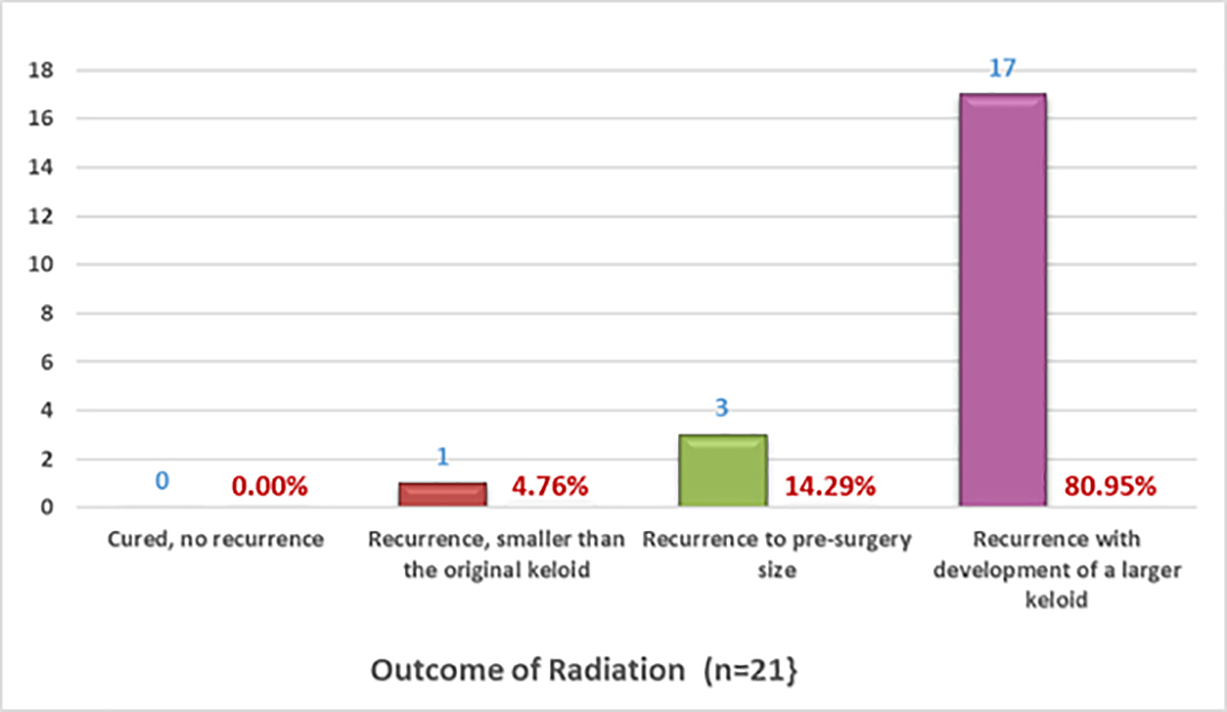
FIGURE 12: Recurrence rates after surgery and radiation therapy on solitary keloids as self-reported by patients.
DISCUSSION
Surgery is a common treatment modality for the removal of keloid lesions; however, it carries an inherent risk of worsening the instant keloid it intends to treat. Tirgan has previously reported that prior keloid removal surgery was the most important risk factor for the development of massive and semi-massive ear keloids [16]. In a case series of 283 patients with ear keloids, 100% of 31 patients with massive and semi-massive ear keloid (P <.0001) and 72% of 181 patients with large ear keloids (P <.0001) had previously undergone keloid removal surgery.
Tirgan has reported similar findings in patients with neck keloids [17]. Among 82 consecutive patients with neck keloids who were seen in a keloid specialty medical practice, 31 had massive or very large neck keloids, 28 of whom had previously undergone at least one prior keloid removal surgery (P < 0.0001). Despite physicians’ common practice of recommending surgery to many keloid patients, the data from this study indicate that less than 5% of study participants found surgery to be curative and over 78% reported no benefit from surgery. Most importantly, this study revealed that surgery can be harmful and cause worsening of keloids in 56.20% of all patients undergoing excision of their keloid.
Alternative Treatments for Early-Stage Keloid Lesions
Several reports, some dating back to 1993 [15], point to potential harm due to keloid surgery; therefore, the treatment approach to early-stage keloid lesions should be reevaluated. Since all keloids start as a small papule or minor linear lesion that gradually grows to a size when treatment is considered, it is critical to identify and treat all early-stage keloid lesions with aggressive nonsurgical methods. Such approaches would include early intervention with intralesional steroids, intralesional chemotherapy, and contact cryotherapy [19].
As a general rule and as an accepted and basic principle of medicine, treating any illness is easier when the illness is at its earliest stages. The same principle applies to keloid lesions; the treatment of which with aggressive nonsurgical methods in the early stages will facilitate early and better control over the KD burden. Although the surgical treatment of keloids can lead to a cure in some patients, surgery clearly carries an unacceptably high risk of harming patients by the worsening and formation of much larger keloids. Therefore, the correct approach to early-stage keloid lesions is to treat them very aggressively with nonsurgical methods to achieve complete remission in the shortest time. It is only with this approach that we can have a substantial impact on the natural history of this disorder [18].
Although a nonsurgical approach for the treatment of early-stage keloids may not prove to be curative for all patients, it will help a great majority of patients and prevent the development of massive and life-changing keloids that are triggered solely by surgical intervention. The rates of 9.4% and 56.20% of keloid worsening reported by Escarmant et al. and the present study, respectively, are clearly unacceptable.
Utility of Cryotherapy as an Alternative to Surgery
The use of cryotherapy in keloid treatment was first reported by Allington in 1950 [19]. The first large-scale and systematic review of cryotherapy in the treatment of keloids was published by Muti (1983) who reported on their experience with 100 patients with keloids and concluded that “cryotherapy produced excellent results in the treatment of both keloids and hypertrophic scars; thus, it can be regarded as more effective and less dangerous than intralesional triamcinolone, compression, surgery, and radiation therapy for keloid treatment” [20].
Fikrle (2005) reported the efficacy of cryotherapy as monotherapy in the treatment of earlobe keloids in seven patients aged 9–22 years [21]. The keloid volume was reduced in all cases, complete flattening was observed in five patients, and a pronounced reduction to a maximum of 25% of the previous thickness in another patient. One patient discontinued the therapy because of pain after only partial improvement. The procedure was painful for all patients; however, no further side effects were noticed. No recurrence was observed within 1–4.5 years of the follow-up. Fikrle concluded that cryotherapy had an excellent effect as monotherapy for treating earlobe keloids in young patients.
Van Leeuwen and Huang (2015) studied the application of intralesional cryotherapy in 20 keloid lesions [22, 23]. Nine lesions received a second treatment 6 months after the first treatment. The authors concluded that intralesional cryotherapy showed favorable results in terms of volume reduction, pain, and pruritus, but it did not completely remove the keloids in all the patients. The same group performed a randomized study to compare the outcomes of intralesional cryotherapy with surgery; however, they were unable to accrue enough number of participants, and therefore, stopped their study after randomizing 26 patients.
Schwaiger et al. (2017) studied the therapeutic efficacy of combining cryotherapy with intralesional triamcinolone [24]. Fifteen patients (seven women, eight men) aged 18–54 years with medium-sized keloids, which had existed for an average of 7.8 years, were included in their study. The patients mostly had upper torso keloids, including on the shoulder, breast, and back, and one patient had a neck keloid. The patients received four sessions of cryotherapy, which was directly followed by intralesional injections of triamcinolone acetonide into each keloid at monthly intervals. After the first treatment session and before the second one, the elevation of the lesion was significantly reduced by 18.3% (615.1%) (p = .0143) compared with the baseline elevation. Subsequent documentation showed a reduction of 29.9% (617.9%) (p = .2258) after the second, 37.8% (619.9%) (p = .3391) after the third, and 41.3% (620.6%) (p = .2075) after the fourth treatment session compared with the baseline elevation.
Yosipovitch (2001) conducted a controlled study to evaluate the combined effect of intralesional corticosteroid injection and cryotherapy vs. intralesional corticosteroid or cryotherapy alone [25]. A total of 14 patients (aged 17–50 years) with at least two keloids of 1 year or more in duration on the chest, back, neck, and arms participated in the study after providing informed consent. They received intralesional corticosteroid injections of triamcinolone acetate with and without cryotherapy or only cryotherapy for at least three sessions with intervals of 4 weeks. Overall, nine male and one female patient completed the study (eight patients were Chinese and two were Malays). The authors concluded that the combined treatment with triamcinolone and cryosurgery may be an effective treatment for flattening keloids. The results achieved with the combination therapy were much better than those with triamcinolone injections alone.
Muthanna (2020) reported successfully treating bilateral earlobe keloids in one patient using a cryogun and the spray method [26].
The review of the aforementioned studies revealed that each author used a different cryotherapy method. Muti performed contact cryotherapy using an apparatus that circulated nitrous oxide inside a probe. The probe was then applied to the keloid lesion [20]. Van Leeuwen used liquid nitrogen that circulated inside an open-ended probe, which was inserted inside the keloid lesion, a method that is marketed as intralesional cryotherapy [22]. Schwaiger used a spray gun to spray liquid nitrogen on the surface of the keloids [24]. Regardless of the method uses to deliver cryotherapy, the treatment was found to be efficacious, especially in combination with intralesional triamcinolone.
The survey also queried participants about prior cryotherapy experience and its effect on their keloids. A total of 136 participants reported having had prior cryotherapy. A total of 130 participants provided information regarding the location of their keloids. Participants who had multiple keloids were asked to indicate all the sites of disease involvement. The upper chest, shoulder, upper arm, lower chest, pelvis, and earlobe were the most frequently involved areas among this subpopulation.
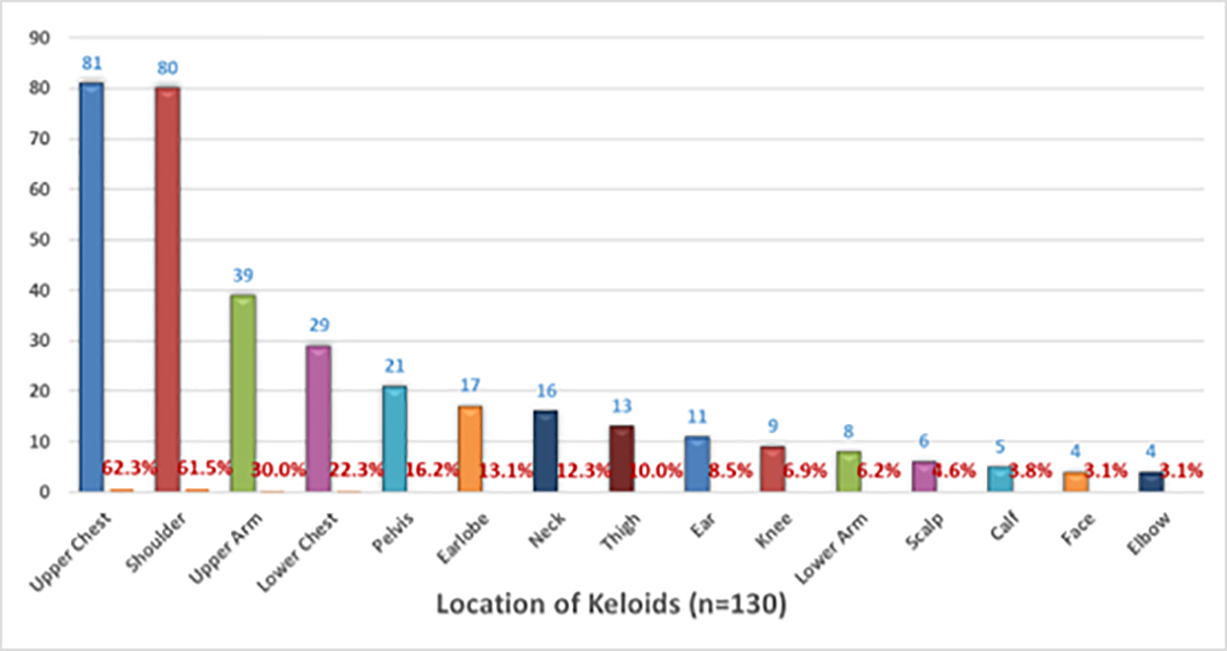
FIGURE 13: Pattern of distribution of keloid lesions among study participants with solitary keloid.
Efficacy of Cryotherapy Among the Survey Participants
The efficacy of cryotherapy was assessed by asking participants to choose only one option for each of the two sets of multiple-choice questions that explored the observed efficacy and overall assessment of benefit from cryotherapy. Figures 14 and 15 depict the responses of the 105 participants who answered the questions about their observed response to cryotherapy and 117 who responded to the questions about the benefit of cryotherapy, respectively.
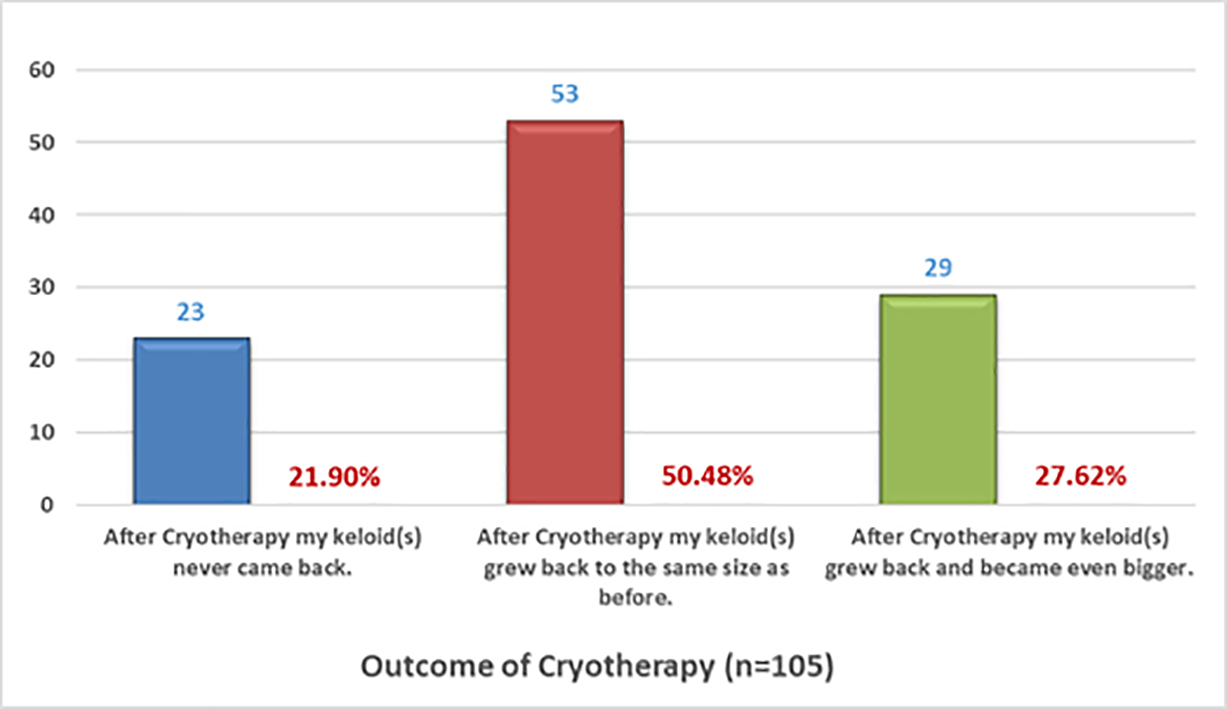
FIGURE 14: Recurrence rates after cryotherapy as self-reported by patients.
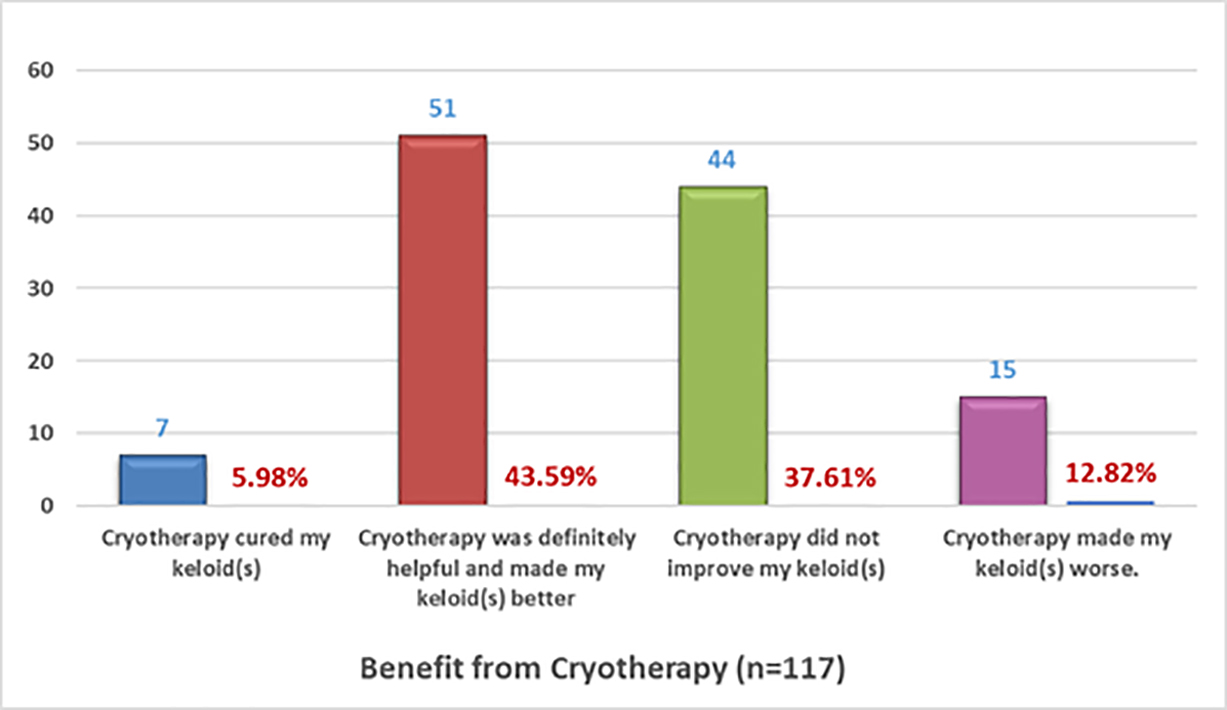
FIGURE 15: Benefit received from cryotherapy as self-reported by patients.
Interestingly, among the patients who self-reported about the results of cryotherapy, a much smaller proportion reported worsening of their keloids (12.82%) compared with those who underwent surgical treatment for their keloids (56.20%).
Case Study
A 24-year-old African American man presented to the author in February 2017 with massive neck keloids. He was asked to produce old photographs that would depict his neck keloids before his first surgery. Figure 16 depicts his neck keloids in December 2014.
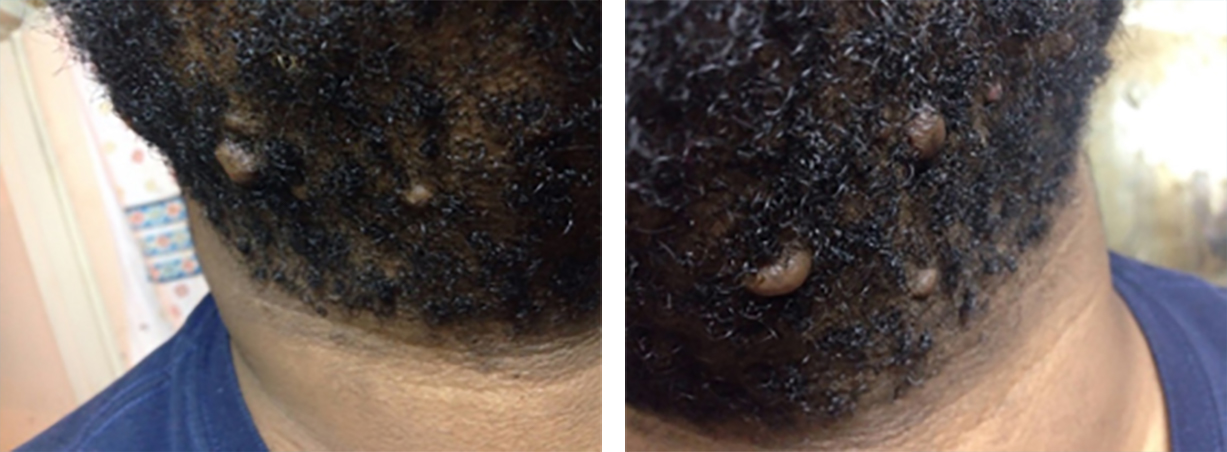
FIGURE 16: December 2014, before undergoing surgery. Photographs from patient’s archives.
He underwent surgical excision of his small neck keloids shortly after these images were taken. Recurrent disease was noted soon after the surgery. Repeat surgery was performed 6 months later to remove all the recurrent keloids, followed by adjuvant intralesional steroid injections. Unfortunately, this approach led to recurrence and much faster growth and eventually the development of massive submental keloids (Figure 17).
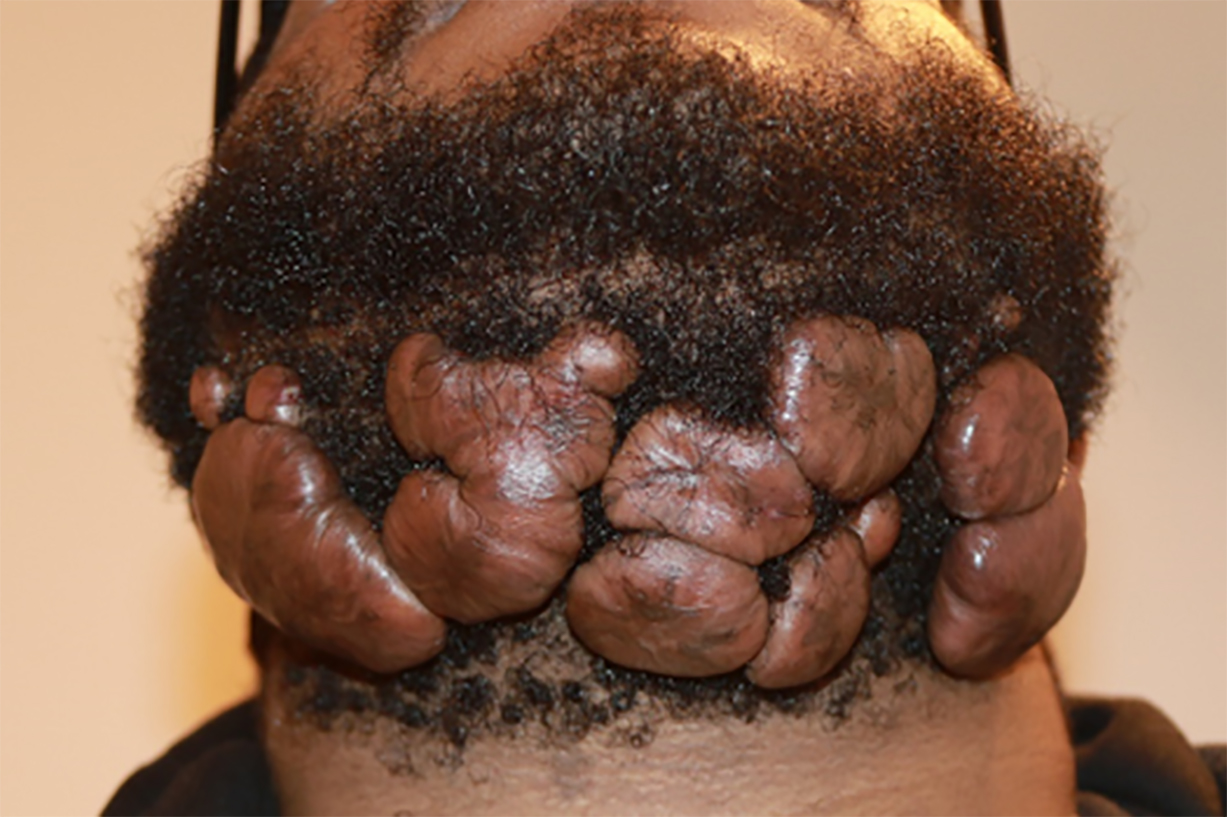
FIGURE 17: A 24-year-old African American man presented in February 2017 with multifocal, massive neck keloids following surgical removal of small neck keloids and intralesional steroid injection.
Unfortunately, many such young patients undergo surgery hoping for a quick resolution of their keloids. However, as exemplified in this case, keloid removal surgery imposes a major risk for far worse outcomes. Although the outcome of this patient’s neck keloids had he not undergone the two surgeries cannot be known, it would be extremely unusual for small keloid nodules to grow as rapidly as they did in this case.
Loss of Normal Tissue
An unreported outcome of keloid removal surgery, especially in patients with ear keloid, is disfigurement, and loss of normal ear tissue. In the present paper, the surgical literature reviewed in the Introduction did not mention the loss of normal ear tissue or disfigurement of ears after keloid removal surgery. Figure 18 depicts two such cases.
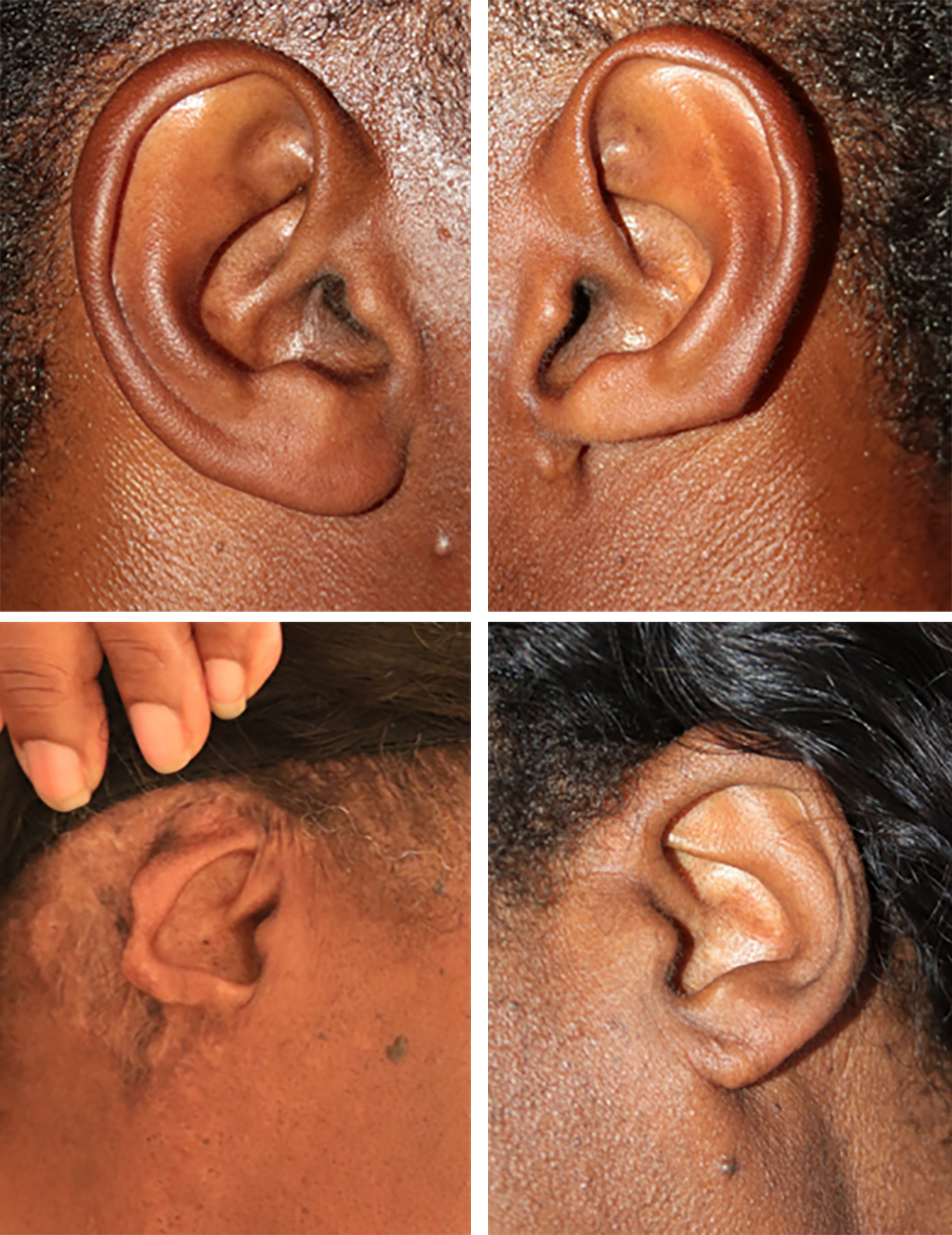
FIGURE 18: Loss of ear tissue following ear keloid surgery and comparison to the opposite ear without loss of tissue, an unreported outcome.
Flaws in the Keloid Literature
Another trend, highlighted earlier in this paper, is the frequent quotation of secondary sources. For example, of the four publications in which the worsening of keloids after surgery was mentioned [5,6,9,10], none of the authors provided original evidence for this statement. Additionally, none referenced Escarmant et al. (1993), who reported the rate of worsening among their own patients [15], which was the only original, level 2 evidence that supports this claim.
The reference to the recurrence rate of keloids after surgery is a frequently quoted review by Mustoe (2002), who stated, “excision alone of keloids results in a high rate of recurrence (45%–100%)” [1]. Mustoe, however, did not provide original data, instead citing Berman et al. [27], Darzi et al. [28], Lawrence [29], and Berman et al. [30].
Berman et al. (1996) published a review of literature on adjunct therapies to surgical management of keloids [27]. This publication did not provide any primary research or data; the authors instead relied on two prior publications, one by Lawrence [29] and another one by Edsmyr et al. [31], when they stated that “excisional surgery of keloids with a scalpel, in the absence of adjunctive therapy, results in 45%–100% recurrence rate.”
The second paper referenced by Mustoe was published by Darzi et al. (1992), who reported the results of their experience in treating 65 keloid patients with 100 keloids on four different treatment protocols [28]. The authors did not treat any patients with surgery alone, and it appears that this paper has no relevance to the subject matter of keloid recurrence after surgery.
The third paper referenced by Mustoe was published by Lawrence (1991), who reported 70% recurrence of keloids treated with surgery followed by adjuvant triamcinolone injections, and 68% recurrence of keloids treated with surgery followed by postoperative colchicine, among patients who were treated on a research protocol [29].
The literature review by Lawrence, however, summarized postoperative recurrence data published by other researchers, wherein reference was made to Conway et al. (1960) who reported a cure rate of 55% (or 45% recurrence) after treating 28 patients with keloid who underwent excision alone [32]. This appears to be the publication reporting 45% recurrence in a cohort of patients with keloid treated with surgery alone.
The fourth paper cited by Mustoe was published by Berman et al. (1995), in which the authors only summarized the literature and quoted a recurrence rate of 45%–100% after surgery without providing a reference to this recurrence rate [30].
The publication by Edsmyr et al. (1974) that was quoted by Berman reported their experience of 174 keloid patients from East Africa. According to the authors, all patients had “serious symptoms from keloids” [31]. In their series, the authors followed 12 patients who had undergone surgery alone without any additional adjuvant therapies and reported 100% recurrence among these 12 patients. The rest of their patients were treated with adjuvant radiation therapy. The authors did not provide any details about the extent of KD in these 12 patients. This seems to be the only publication reporting 100% recurrence in a cohort of keloid patients treated with surgery alone.
Diverse Biology of KD
There is, however, an inherent and more important flaw in all the treatment outcome reports. This flaw applies to the biology of the underlying KD among study patients. Clinical observation has taught us that not all patients have biologically similar diseases. In our day-to-day practices, we see a wide range of keloid patients, from those who present with only one keloid lesion to those with widespread disease involving many areas of their skin. We also see a range of time courses, with some patients having quite slow-growing keloids and others who develop numerous large keloids over a short period. This concept was not discussed in any of the literature reviewed.
Biology and genomics play a significant role in the treatment outcomes in many disease entities [33], such as breast cancer [34], lung cancer [35], colon cancer [36], rheumatoid arthritis [37], and hemophilia [38], and KD is not excepted, as it has diverse and highly variable clinical presentations and behavior, all of which may affect treatment outcomes.
Next-generation sequencing of tumor tissue can guide clinical management by providing information about actionable gene aberrations that have diagnostic and therapeutic significance [33]. It is therefore plausible that the biology of the underlying KD also plays a role in the treatment outcomes, a concept that should be incorporated into future keloid research studies.
Tirgan and Kerby (2021) conducted a detailed analysis of two large datasets of patients with KD and showed that KD has a diverse clinical behavior with variable rates of growth over time [39]. Clinical dataset analysis of 971 patients with KD confirmed that 8.02% of patients had stable disease and remained in stage I for ≥15 years. Analysis of a separate survey dataset of 1709 patients with keloid showed that, in 35% of this large group, the disease had not progressed in 10 years.
Like many other illnesses, KD is not a homogeneous disease. Its highly variable biology may account for the wide range of recurrence rates among patient cohorts. Furthermore, most authors do not report the location of the excised keloids, growth rate, keloid formation duration, triggering factors leading to keloid formation, absence of keloids elsewhere, prior treatments, or the KD stage. All these factors have been indirectly tied to the biology of the underlying KD and can potentially influence the postoperative rate of recurrence.
LIMITATIONS
The present study has several limitations. The enrollment process might have been biased toward those searching the internet for information related to their illness, or subjects exploring treatment options for their recurrent keloids, with possible overrepresentation of younger and more computer-literate individuals. Since the survey was conducted in English, it excluded non-English-speaking individuals. The survey did not collect data regarding the timing and other specific details of the surgery or the adjuvant treatment modalities that the patients might have received.
The survey was not designed, nor was it able, to compare the treatment outcomes of the different treatment modalities. This is partially due to the fact that different types of keloids require different treatment methods. Real-world experience suggests that most ear keloids are excised with surgery. This survey purely reflects this real-world experience, whereby a large nodular keloid is not treated with lasers or with steroid injections. Moreover, surgery is often not performed for the treatment of multifocal chest/shoulder keloids or multifocal scalp keloids. As a result, the survey cannot be used to answer comparative questions among all patients with keloid.
As for cryotherapy, the author is unaware of any randomized studies that have compared the outcome of surgery with cryotherapy in treating keloids. Therefore, any comparison made between these modalities will be speculative and not based on solid evidence.
Finally, the survey tool was not validated. Despite these limitations, this self-selected group of respondents provides a glimpse into the real-world experience of those with keloids, including their perceptions regarding the efficacy of surgery, which is commonly offered to community-dwelling patients with keloids.
Each of the above limitations has a potential to influence and skew the study results. However, the main aim of this study is to highlight the finding of potential harm from keloid surgery, something that is not reported in surgical research. However, information obtained from a retrospective survey tool can never be as robust as the information obtained from a prospective clinical study.
CONCLUSION
Among the survey participants, surgery was found to be curative in less than 5% of patients and led to the worsening of keloids in 56.20% of patients.
Physicians who treat keloid patients should avoid recommending surgery, especially in patients with early-stage keloids, and maximize the use of nonsurgical modalities such as cryotherapy and intralesional chemotherapy. The Keloid Research Foundation has published a comprehensive set of guidelines for the treatment of keloid patients as well as guidance documents for proper usage of intralesional treatments and cryotherapy [40].
Based on the results of this large dataset, it is best to validate the actual treatment outcomes in patients with newly diagnosed ear keloid(s) in a randomized or partially randomized study whereby the short- and long-term outcomes of the primary surgery followed by any form of adjuvant treatment, including adjuvant radiation therapy, is compared with the outcomes of primary cryotherapy.
Finally, those who continue to perform surgery on keloid patients should consider reporting the rate of worsening of keloids and the rate of ear disfigurement among patients with ear keloids. Patient-reported outcomes should be prospectively integrated with all keloid treatment studies. Investigators should work together to design and validate reliable tools that can be incorporated into the designs of future clinical keloid studies so that the benefits and potential risks of surgery and all other treatment modalities can be more accurately assessed.
Conflict of interest disclosure
The author has no conflicts of interest to disclose.
ORCiD
Michael Tirgan https://orcid.org/0000-0002-4761-403X
REFERENCES
- Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571.
- Gold MH, Nestor MS, Berman B, Goldberg D. Assessing keloid recurrence following surgical excision and radiation. Burns Trauma. 2020 Nov 14;8:tkaa031. doi: 10.1093/burnst/tkaa031. PMID: 33225004; PMCID: PMC7666880.
- Ogawa R, Dohi T, Tosa M, Aoki M, Akaishi S. The Latest Strategy for Keloid and Hypertrophic Scar Prevention and Treatment: The Nippon Medical School (NMS) Protocol. J Nippon Med Sch. 2021 Mar 11;88(1):2-9. doi: 10.1272/jnms.JNMS.2021_88-106. Epub 2020 Aug 1. PMID: 32741903.
- Lemperle G, Schierle J, Kitoga KE, Kassem-Trautmann K, Sachs C, Dimmler A. Keloids: Which Types Can Be Excised without Risk of Recurrence? A New Clinical Classification. Plast Reconstr Surg Glob Open. 2020 Mar 27;8(3):e2582. doi: 10.1097/GOX.0000000000002582. PMID: 32537319; PMCID: PMC7253266.
- Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. The Keloid Disorder: Heterogeneity, Histopathology, Mechanisms and Models. Front Cell Dev Biol. 2020 May 26;8:360. doi: 10.3389/fcell.2020.00360. PMID: 32528951; PMCID: PMC7264387.
- Robles, D., and Berg, D. (2007). Abnormal wound healing: keloids. Clin. Dermatol. 25, 26–32.
- Butler, P. D., Ly, D. P., Longaker, M. T., and Yang, G. P. (2008b). Use of organotypic coculture to study keloid biology. Am. J. Surg. 195, 144–148. doi: 10.1016/j. amjsurg.2007.10.003
- Balci, D., Inandi, T., Dogramaci, C., and Celik, E. (2009). DLQI scores in patients with keloids and hypertrophic scars: a prospective case control study. J. Dtsch. Dermatol. Ges. 7, 688–692. doi: 10.1111/j.1610-0387.2009.07034.x
- Shih, B., Garside, E., McGrouther, D. A., and Bayat, A. (2010). Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 18, 139–153. doi: 10.1111/j.1524-475X.2009.00553.x
- Poochareon VN, Berman B. New Therapies for the Management of Keloids. Journal of Craniofacial Surgery. 2003;14(5):654-657.
- Shaffer JJ, Taylor SC, Cook-Bolden F. Keloidal scars: a review with a critical look at therapeutic options. J Am Acad Dermatol 2002;46(Suppl):S63
- Bijlard E, Timman R, Verduijn GM, et al. Intralesional cryotherapy versus excision with corticosteroid injections or brachytherapy for keloid treatment: randomised controlled trials. J Plast Reconstr Aesthet Surg. 2018; 71:847–856.
- Sasidharan A, David A, Gohil A, Gupta AK. Simple device to determine the pressure applied by pressure clips for the treatment of earlobe keloids. Indian J Plast Surg 2015;48:293–6.
- Bennett, K. Kung, T. Hayman, J. Brown, D. & (2017). Treatment of Keloids With Excision and Adjuvant Radiation. Annals of Plastic Surgery, 78 (2), 157-161. doi: 10.1097/SAP.0000000000000903.
- Escarmant P, Zimmermann S, Amar A, Ratoanina JL, Moris A, Azaloux H, Francois H, Gosserez O, Michel M, G'Baguidi R. The treatment of 783 keloid scars by iridium 192 interstitial irradiation after surgical excision. Int J Radiat Oncol Biol Phys. 1993 May 20;26(2):245-51. doi: 10.1016/0360-3016(93)90204-9. PMID: 8491682.
- Tirgan M. Massive ear keloids: Natural history, evaluation of risk factors and recommendation for preventive measures–A retrospective case series [version 2; peer review: 1 approved, 2 approved with reservations]. F1000Research 2017, 5:2517 (https://doi.org/10.12688/f1000research.9504.2)
- Tirgan MH. Neck keloids: evaluation of risk factors and recommendation for keloid staging system [version 2; peer review: 2 approved]. F1000Research 2016, 5:1528 (https://doi.org/10.12688/f1000research.9086.2)
- Tirgan MH, Clinical Practice Guidelines in Keloid Disorder (KRF Guidelines®) Treatment Strategy - Version 1.2019, Mar. 10, 2019. Journal of Keloid research. Special Edition, April 1, 2019
- Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950 Mar;72(3):153-5. PMID: 15410141; PMCID: PMC1520330.
- Muti E, Ponzio E. Cryotherapy in the treatment of keloids. Ann Plast Surg. 1983 Sep;11(3):227-32. doi: 10.1097/00000637-198309000-00009. PMID: 6638822.
- Fikrle, T. & Pizinger, K. (2005). Cryosurgery in the Treatment of Earlobe Keloids. Dermatologic Surgery, 31 (12), 1728-1731.
- van Leeuwen, M. C. van der Wal, M. B. Bulstra, A. Galindo-Garre, F. Molier, J. van Zuijlen, P. P. van Leeuwen, P. A. & Niessen, F. (2015). Intralesional Cryotherapy for Treatment of Keloid Scars. Plastic and Reconstructive Surgery, 135 (2), 580-589. doi: 10.1097/PRS.0000000000000911.
- Huang, J. Yu, N. & Wang, X. (2015). Intralesional Cryotherapy for Treatment of Keloid Scars. Plastic and Reconstructive Surgery, 136 (3), 394e-395e. doi: 10.1097/PRS.0000000000001492.
- Schwaiger, H. Reinholz, M. Poetschke, J., Ruzicka, T. & Gauglitz, G. (2018). Evaluating the Therapeutic Success of Keloids Treated With Cryotherapy and Intralesional Corticosteroids Using Noninvasive Objective Measures. Dermatologic Surgery, 44 (5), 635-644. doi: 10.1097/DSS.0000000000001427.
- Yosipovitch G, Widijanti Sugeng M, Goon A, Chan YH, Goh CL. A comparison of the combined effect of cryotherapy and corticosteroid injections versus corticosteroids and cryotherapy alone on keloids: a controlled study. J Dermatolog Treat. 2001 Jun;12(2):87-90. doi: 10.1080/095466301317085363. PMID: 12243664.
- Muthanna AM, Al-Qubati YA. Cryotherapy: A Successful Monotherapy for Earlobe Keloids. Malays Fam Physician. 2020 Nov 10;15(3):83-85. PMID: 33329867; PMCID: PMC7735875.
- Berman B, Bieley HC. Adjunct therapies to surgical management of keloids. Dermatol Surg. 1996 Feb;22(2):126-30. doi: 10.1111/j.1524-4725.1996.tb00493.x. PMID: 8608373.
- Darzi MA, Chowdri NA, Kaul SK, Khan M. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. British journal of plastic surgery. 1992;45(5):374-379. doi:10.1016/0007-1226(92)90008-L
- Lawrence WT. In search of the optimal treatment of keloids: report of a series and a review of the literature. Annals of plastic surgery. 1991;27(2):164-178. doi:10.1097/00000637-199108000-00012
- Berman B, Bieley HC. Keloids. Journal of the American Academy of Dermatology. 1995;33(1):117-123. doi:10.1016/0190-9622(95)90035-7
- Edsmyr F, Larsson LG, Onyango J, Wanguru S, Wood M. Radiation Therapy in the Treatment of Keloids in East Africa. Acta oncologica. 1974;13(2):102-106. doi:10.3109/02841867409129869
- Conway H, Gillette R, Smith JW, Findley A. Differential diagnosis of keloids and hypertrophic scars by tissue culture technique with notes on therapy of keloids by surgical excision and decadron. Plastic and reconstructive surgery and the transplantation bulletin. 1960;25:117-132.
- Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019 Apr;110(4):1480-1490. doi: 10.1111/cas.13969. Epub 2019 Apr 2. PMID: 30742731; PMCID: PMC6447843.
- Sultova E, Westphalen CB, Jung A, Kumbrink J, Kirchner T, Mayr D, Rudelius M, Ormanns S, Heinemann V, Metzeler KH, Greif PA, Hester A, Mahner S, Harbeck N, Wuerstlein R. Implementation of Precision Oncology for Patients with Metastatic Breast Cancer in an Interdisciplinary MTB Setting. Diagnostics (Basel). 2021 Apr 20;11(4):733. doi: 10.3390/diagnostics11040733. PMID: 33924134; PMCID: PMC8074310.
- Bonanno L, Pavan A, Ferro A, Calvetti L, Frega S, Pasello G, Aprile G, Guarneri V, Conte P; Rete Oncologica Veneta (ROV). Clinical Impact of Plasma and Tissue Next-Generation Sequencing in Advanced Non-Small Cell Lung Cancer: A Real-World Experience. Oncologist. 2020 Dec;25(12):e1996-e2005. doi: 10.1634/theoncologist.2020-0148. Epub 2020 Jul 7. PMID: 32557976; PMCID: PMC8108051.
- Pritzker KPH. Colon Cancer Biomarkers: Implications for Personalized Medicine. J Pers Med. 2020 Oct 13;10(4):167. doi: 10.3390/jpm10040167. PMID: 33066312; PMCID: PMC7711712.
- Cherlin S, Lewis MJ, Plant D, Nair N, Goldmann K, Tzanis E, Barnes MR, McKeigue P, Barrett JH, Pitzalis C, Barton A; MATURA Consortium, Cordell HJ. Investigation of genetically regulated gene expression and response to treatment in rheumatoid arthritis highlights an association between IL18RAP expression and treatment response. Ann Rheum Dis. 2020 Nov;79(11):1446-1452. doi: 10.1136/annrheumdis-2020-217204. Epub 2020 Jul 30. PMID: 32732242; PMCID: PMC7569378.
- Berntorp E, Fischer K, Hart DP, et al. Haemophilia. Nature reviews Disease primers. 2021;7(1):45-45. doi:10.1038/s41572-021-00278-x
- Tirgan MH, Kerby E, Cartesian Model of the Clinical Behaviors of Keloid Disorder, Implementation of an Updated Keloid Staging System, and Call for Establishment of an International Keloid Registry. 2021;5(1):11-23
- https://keloidresearchfoundation.org/treatment-guidelines/
METRICS
Surgical treatment of keloid lesions: help or harm?
Michael H. Tirgan, MD
Keloid Research Foundation
23 West 73rd Street, Suite GD, New
York, NY 10065
(212) 874-4200
Tirgan@KeloidResearchFoundation.org
Conflict of Interest
None
Funding
None
Word Count
7171
IRB appRoVal
The online survey was approved by Western IRB
Keywords
Keloid, Surgery
