Cartesian Model of the Clinical Behaviors of Keloid Disorder, Implementation of an Updated Keloid Staging System, and Call for Establishment of an International Keloid Registry
Michael H. Tirgan, MD , Eva Kerby, MD
ABSTRACT
Background: Keloid disorder (KD) has a highly variable clinical behavior, ranging from patients who only develop one or a few keloid lesions in their lifetime to patients who develop extensive disease involving various parts of their skin within a few years. To the authors’ knowledge, this critical aspect of the disorder has yet to be adequately studied and incorporated into the analysis of clinical or laboratory research in KD patients.
Objective: To assess variability in clinical behaviors and rates of progression of KD through clinical data analysis.
Materials and Methods: A retrospective analysis of two large clinical datasets was conducted. The first dataset comprised 1088 consecutive patients seen by the first author in his keloid specialty practice. The first author and co-author each independently analyzed the medical records and photographs of the lesions from all patients. This dataset hereafter will be referred to as the “clinical dataset.” Patterns of clinical progression of KD among these patients were plotted in Cartesian tables. The second dataset was obtained from an ongoing online keloid survey that the first author launched in November 2011. A total of 1,709 participants were asked to provide answers to numerous questions about their keloids, including an assessment of their keloids’ growth rates over time. This dataset hereafter will be referred to as the survey dataset.” Patterns of clinical progression of KD among the survey participants were plotted in several graphs
The Institutional Review Board (IRB) approved the underlying studies for both
datasets. Descriptive statistics are provided.
Results: After review of the clinical patterns of presentation and duration of KD, the patients’ results were tabulated in Cartesian tables, and patients with similar patterns of disease behavior were clustered into separate groups.
Among 971 patients in the clinical dataset who met the entry criteria and were analyzed by the first author, 508 (52.32%) patients had stage I disease, 308 (31.72%) had stage II, 115 (11.84%) had stage III, and 40 (5.15%) had stage IV. Seventyeight (8.02%) patients had stage I KD >15 years after disease onset. Additionally, 52 (5.35%) patients developed stage II disease, 15 (1.54%) developed stage III, and four (0.41%) developed stage IV ≤3 years of disease onset. Similar findings were observed by the co-author. Among 976 patients who met the entry criteria, 86 (8.81%) had stage I KD >15 years since disease onset, 42 (4.30%) developed stage II disease, 13 (1.33%) developed stage III, and three (0.31%) developed stage IV ≤3 years of disease onset.
In the survey dataset, approximately one third of the participants reported having stable disease and no progression of KD at 1-, 2-, 5-, and 10-year timepoints. About 10% of the patients reported a 50% increase, and approximately 6% reported doubling of their keloids size at 1, 2, 5, and 10-year timepoints.
Regarding the treatment response and reduction in keloids size, only 6% of the survey participants reported some degree of reduction in the size of their keloids at the 1-, 2-, and 5-year timepoints and about 8% at the 10-year timepoint.
Conclusions and Relevance: Proper comparison of clinical and/or laboratory outcome data is meaningful only among patients with similar disease biology and clinical behavior. The authors recommend that keloid researchers incorporate the variability in clinical behavior of KD in planning for clinical or laboratory experiments and in data analysis. All keloid researchers are invited to collaborate in establishing an international Keloid Registry to meticulously track and understand the clinical behavior of KD.
INTRODUCTION
Learning the natural history of an illness is one of the fundamental steps in understanding the disease process. Treatments are often aimed at altering a disease’s natural history for the better, with the goal of improving patient outcomes. Currently, our understanding of the natural history of KD remains quite limited [1], and our therapeutic interventions often alter KD’s natural history for the worse, causing worsening of keloids and significant harm to our patients. The first author has previously reported on the potential harms from commonly practiced treatment interventions, such as intra-lesional steroids [2], surgery [3,4], and laser treatment[5].
METRICS
Michael H. Tirgan, MD (Corresponding Author)
Keloid Research Foundation
23 West 73rd Street, Suite GD, New
York, NY 10065
(212) 874-4200
htirgan@gmail.com
Eva Kerby, MD
Department of Dermatology, Weill
Cornell Medical College 1305 York Avenue, 9th Floor, New York, NY 10021
(646) 962-3376
evk9019@med.cornell.edu
Manuscript Word Count
4118
Conflict of Interest
None
Funding
None
IRB Assessment
Determined by the Western IRB and
the Weill Cornell IRB to be exempt
from the need for IRB approval.
Keywords
Keloid, Keloid Biology, Keloid
Registry, Keloid Growth
Several groups have attempted to pierce the keloid veil to gain insight into the clinical behavior of this poorly understood disorder. Smith et al. (2013) reported on the rate of spontaneous resolution of keloid lesions among 34 keloid patients who collectively had 126 keloids [6]. The authors observed this phenomenon in 10 (8%) keloid lesions, with a median of 5 years to spontaneous resolution. In addition, Lu et al. (2015) reported on the natural history of the disorder in 715 Chinese keloid patients and concluded that those keloids were characterized by a younger age of onset and weaker female predominance [7]. The most severe forms of keloids among their patients were observed in the probands with a positive family history. Tirgan previously reported on the natural history of massive ear [3] and neck keloids [4], concluding that prior keloid removal surgery is the risk factor for development of massive and semi-massive keloid lesions.
The study aim was to explore patterns of KD progression over time and characterize different patient subgroups with variable biological behaviors. The findings are presented in Cartesian tables highlighting the inherent biological differences among different KD patient cohorts.
MATERIALS AND METHODS
The authors analyzed two separate datasets. The clinical dataset comprised 1088 consecutive patients seen by the first author in his keloid specialty practice between January 1, 2008, and March 27, 2018.
The clinical dataset was initially reviewed by the first author and subsequently by the co-author to cross-check and validate the accuracy of the data. All 1088 patient charts and photographs were reviewed independently and at different times by each author. The age of onset of KD, current patient age, keloid location, prior treatments, and keloid stage (obtained by measurements as shown in Table 1) were verified for each patient.
The inclusion criteria were availability of patient age, reported age at KD onset, gender, ethnic background, KD stage, and a complete set of photographs from all keloid lesions. Only patients with available data for all inclusion criteria were included in the analysis. Patients with incomplete medical records were excluded from this analysis.
Nine hundred and seventy-one (971) patients met the inclusion criteria and were selected for analysis by the first author. The co-author approved and analyzed data from 976 patients. The minor discrepancy in the number of patients included by each author was due to each author’s independent assessment of the available data.
Most patients in this dataset had received a variety of prior treatments. Staging of KD was based on the clinical presentation at the first visit. Disease duration was calculated according to the patients’ recollection of the age when they developed their very first keloid.
The first author had previously introduced a keloid staging system [4] that was used to determine the disease stage for each patient included in this report. When the proposed staging system was applied retrospectively for the first time to this large cohort of patients, it became apparent that it had to be revised. The 2016 staging system differentiated between patients with only one keloid lesion rather than those with multiple keloids. This differentiating factor was subsequently eliminated. The revised keloid staging system (see Table 1) was used to determine the extent of skin involvement based on the sum of the largest diameters of all keloid lesions in each patient.
The authors estimated keloid lesion diameters by visual assessment of the keloid lesion photographs taken during the first patient visit. This assessment only estimated the lesion sizes rather than exactly measuring them because a scale was not included in the photographs of the keloids. Although exact measurements are preferred, estimation of the lesion size was the only method available to the authors and seemed to be adequate for the purpose of categorizing the lesions into various staging groups with wide measurement ranges in each stage category. Figures 1 and 2 depict several examples of stage assignment by estimating the size of keloid lesions in 12 patients.
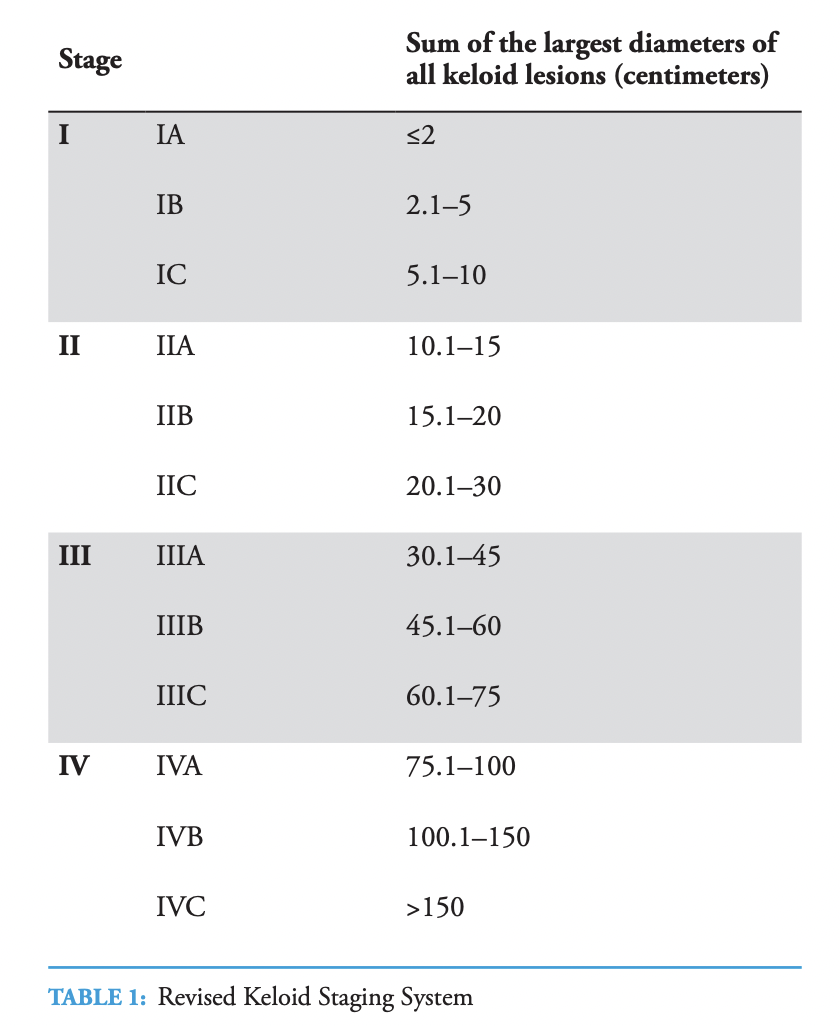
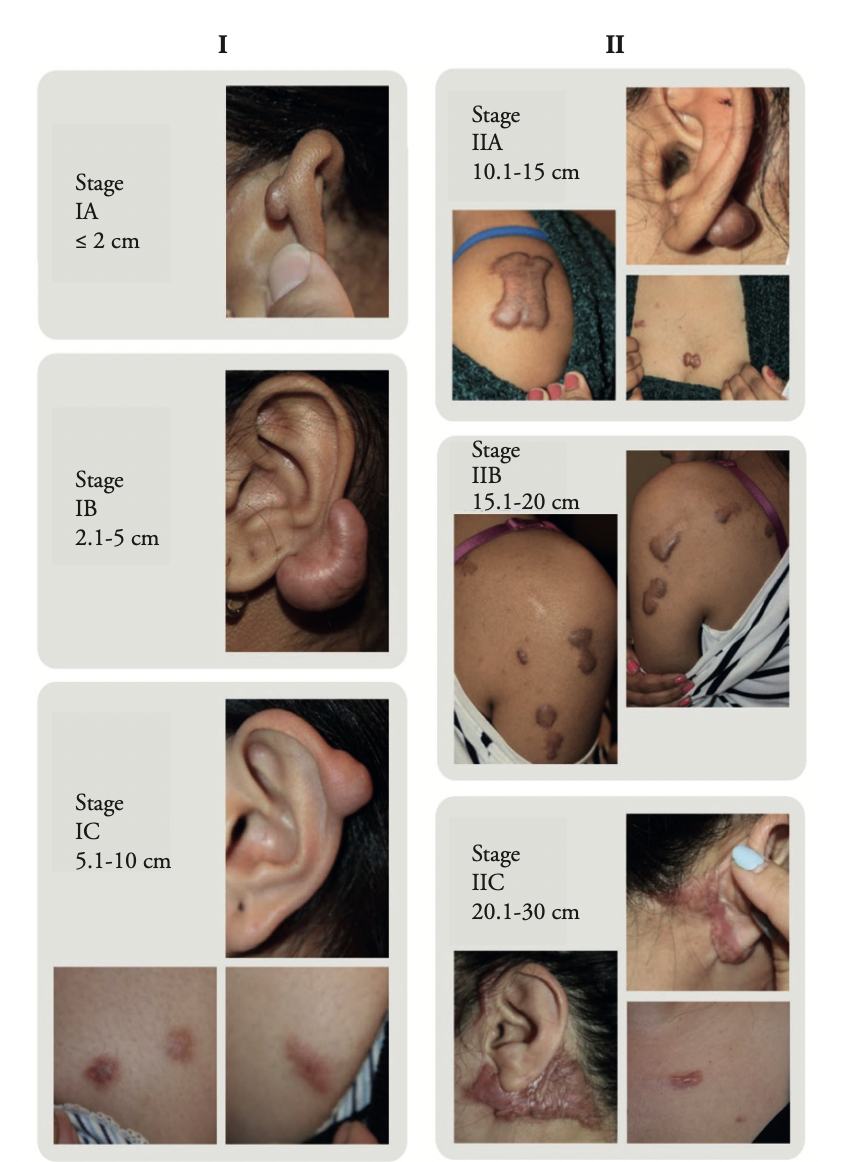
FIGURE 1: Depiction of stages I and II in six patients. Note that the patients depicted with stage IA and stage IB only had one keloid lesion. The remaining patients depicted here had multiple keloids.
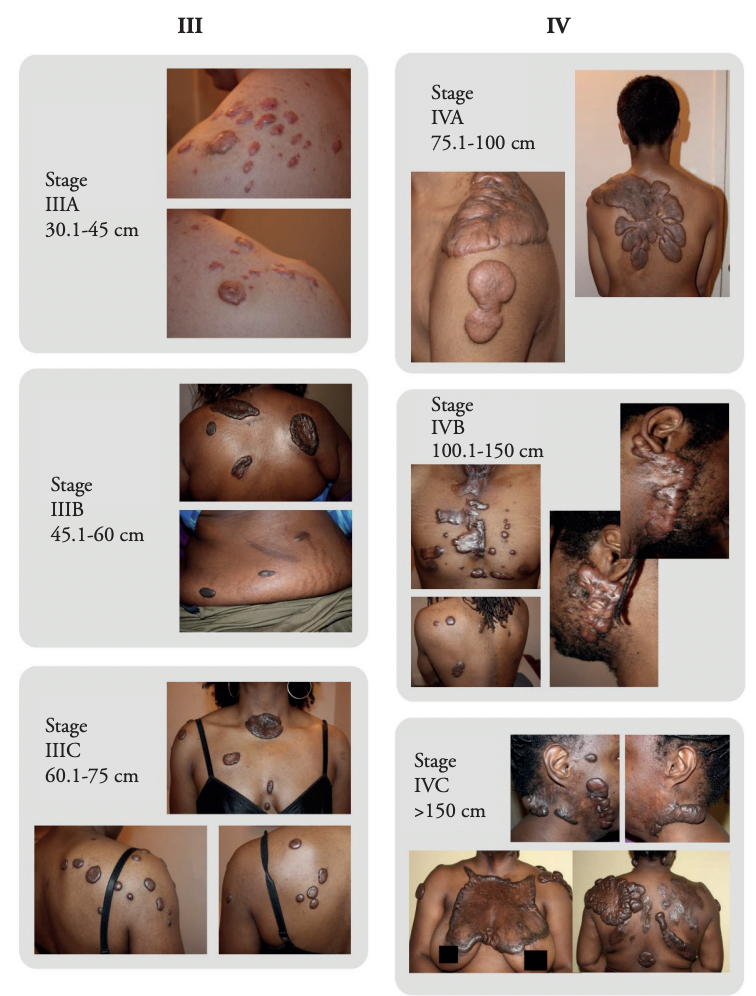
FIGURE 2: Depiction of stages III and IV in six patients.
Disease duration was determined as the time from the date the very first keloid appeared to the date a patient first presented to the first author. This information was available in the medical records of all patients included in this analysis. The patients were grouped in four separate groups based on the duration of their illness (Figures 3 and 4). Each author created a 4 × 4 Cartesian table and assigned each patient to one of 16 subgroups according to disease stage on the Y-axis and duration of illness on the X-axis of the table (Tables 2 and 3).
The first author performed subset analysis of the African American group with KD because this cohort comprised the largest ethnic cohort (n = 524, 53.96%) in the group. Figure 5 and Table 4 depict the same analysis for this subset of patients.
Another resource available to the authors was the survey dataset that was extracted from an online keloid survey that the first author had launched on November 14, 2011. As of November 8, 2019, 1,709 individuals had participated in this survey. As part of this survey, the participants were asked to compare their keloid lesions’ sizes when they took the survey to their sizes 1, 2, 5, and 10 years earlier. Table 5 and graphs 6–9 summarize this data for all survey participants.
The underlying research projects for both datasets were determined by the Western IRB (WIRB Work Order #1- 962226-1, WIRB Work Order #1-963770-1) to meet the conditions for exemption under 45 CFR 46.101(b)(4). The underlying research projects for the clinical dataset were also determined by the Weill Cornell IRB to meet the conditions for exemption under 45 CFR 46.101(b) (4). Consent is not required for studies determined to be exempt under 45 CFR 46.101(b)(4). The IRB records, as well as the research records can be provided upon request
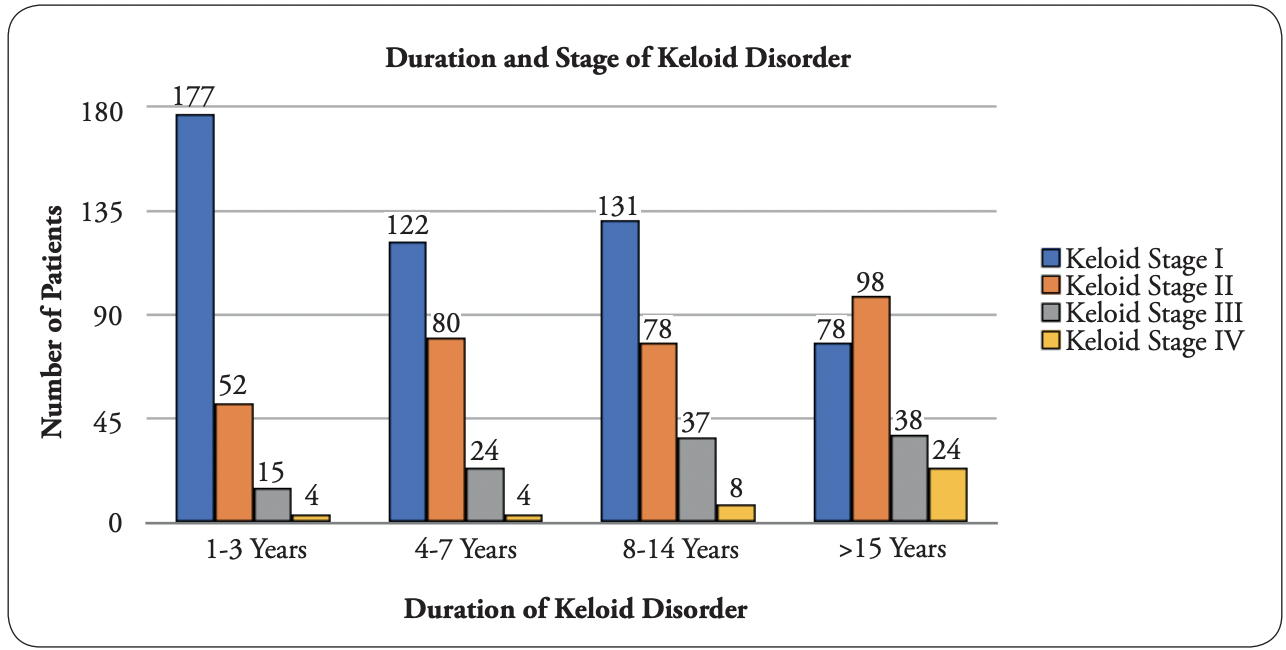
FIGURE 3: Graphic depiction of all patients in the first author’s analysis
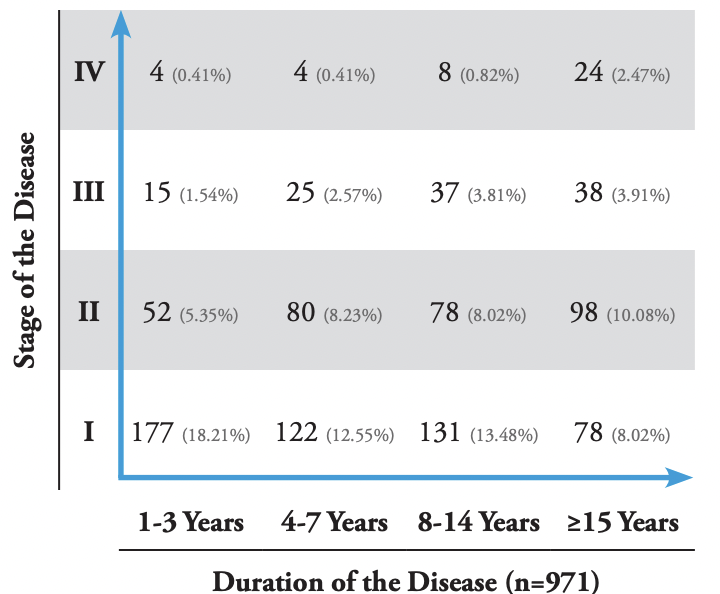
TABLE 2: Cartesian table of keloid disorders from the first author’s analysis.
RESULTS
The clinical dataset comprising 1088 patients was first examined and analyzed by the first author, and 971 patients met the inclusion criteria. Among these 971 patients, 508 (52.32%) had stage I disease, 308 (31.72%) had stage II, 115 (11.84%) had stage III, and 40 (5.15%) had stage IV; additionally, 248 (25.54%) had had their disease for 1–3 years, 231 (23.79%) for 4–7 years, 254 (26.16%) for 8–14 years, and 238 (24.51%) for ≥15 years. Figure 3 shows a summary of this data as a bar graph, and Table 2 presents a summary of this data in a Cartesian table format.
The clinical dataset was subsequently analyzed by the co-author, and 976 patients met the inclusion criteria. Among these 976 patients, 587 (60.14%) had stage I KD, 253 (25.92%) had stage II, 101 (10.35%) had stage III, and 35 (3.59%) had stage IV; additionally, 272
(27.87%) had their disease for 1–3 years, 240 (24.59%) for 4–7 years, 247 (25.30%) for 8–14 years, and 217 (22.24%) for ≥15 years. Figure 4 shows a summary of this data as a bar graph, and Table 3 presents a summary of this data in a Cartesian table format.
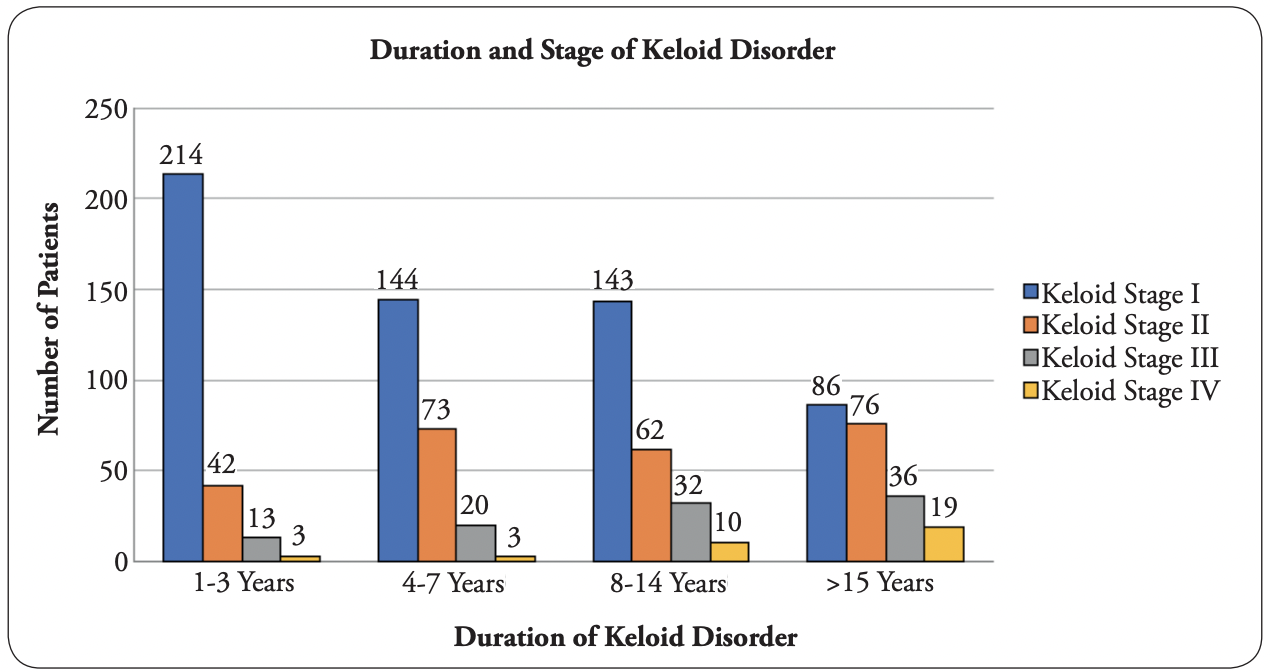
FIGURE 4: Graphic depiction of all patients in the co-author’s analysis.
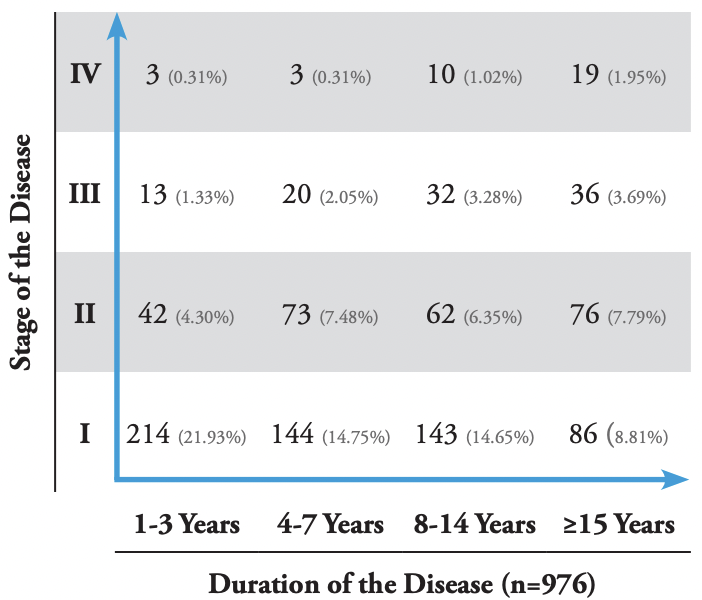
TABLE 3:Cartesian table of keloid disorder, co-author’s analysis.
The first author performed subset analysis on the African American group with KD, as this cohort comprised the largest ethnic cohort (n = 524, 53.96%) in the group. Among these patients, 251 (47.90%) had stage I disease, 176 (33.59%) had stage II, 70 (13.36%) had stage III, and 27 (5.15%) had stage IV. Among these patients, 138 (26.34%) had their disease for 1–3 years, 114 (21.76%) for 4–7 years, 129 (24.62%) for 8–14 years, and 143 (27.29%) for ≥15 years. Figure 5 summarizes this data in a bar graph, and Table 4 summarizes this data in a Cartesian table format.
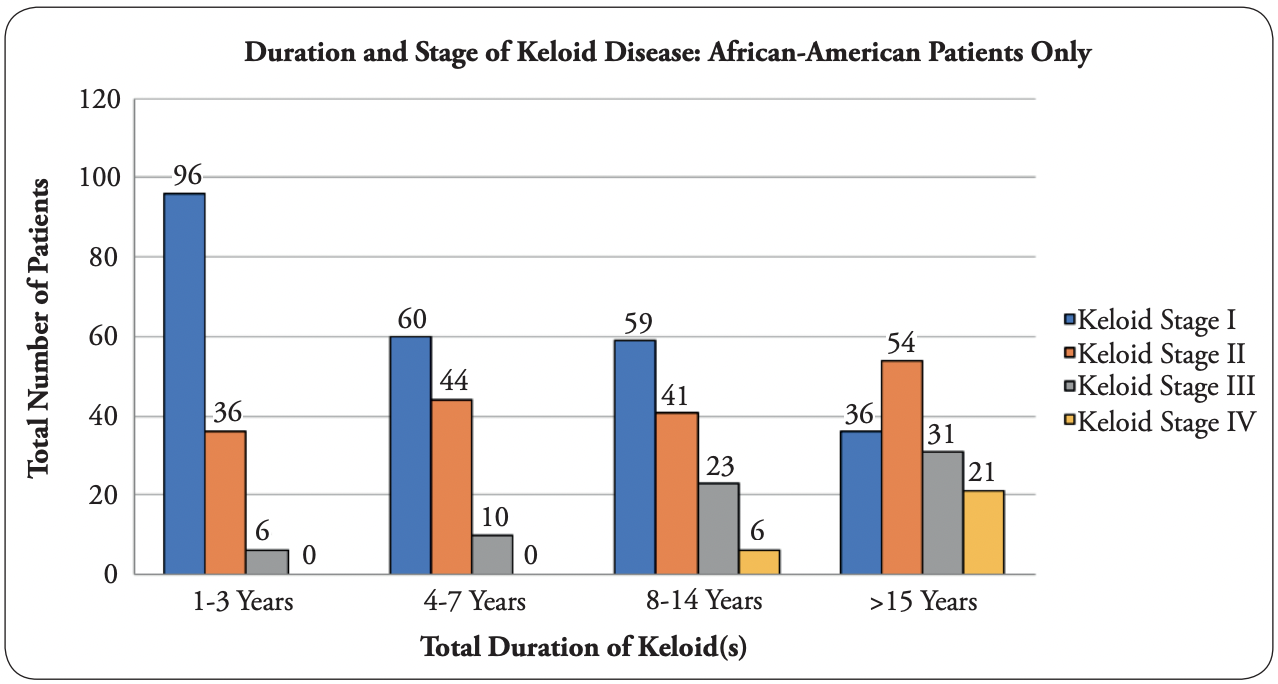
FIGURE 5: Graphic depiction of African American patients only, first author’s analysis
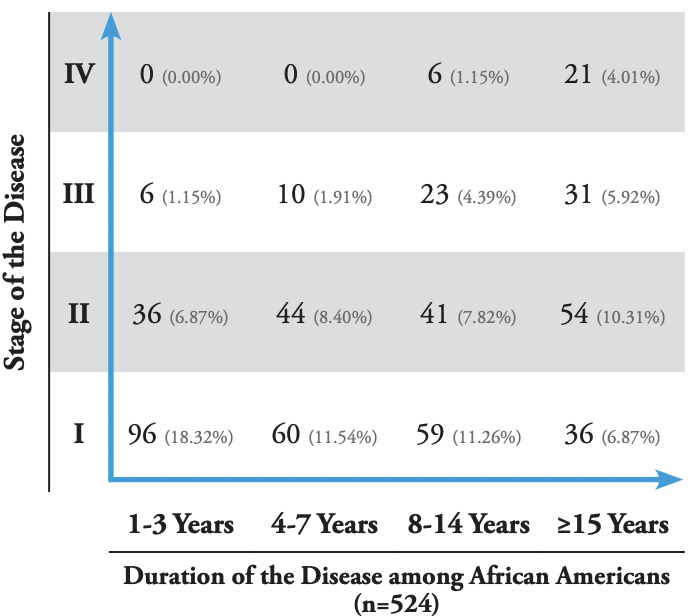
TABLE 4: Cartesian table of keloid disorder, African American patients only, first author’s analysis.
The survey dataset comprised 1,709 participants who took the online keloid survey. Among these participants, 1,356 adults provided answers to the first query that compared the current size of their keloid(s) to their size 1 year earlier; 1,248 participants provided a 2-year comparison; 1,092 provided a 5-year comparison; and 902 participants provided a 10-year comparison. Table 5 provides a raw summary of this data. Figures 6–9 show variable rates of growth of the keloid lesions as reported by the survey participants at different timepoints.
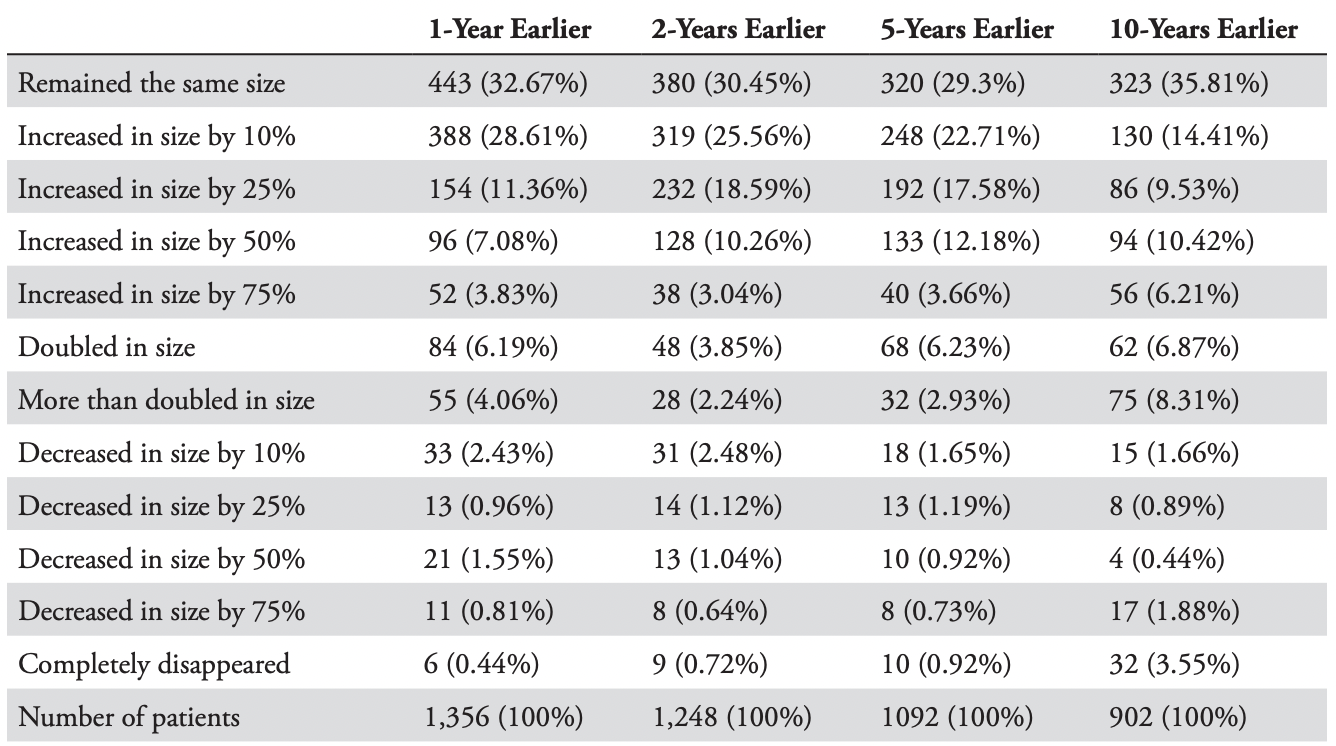
TABLE 5: Summary of growth rates (or regression) among 1,356 survey participants
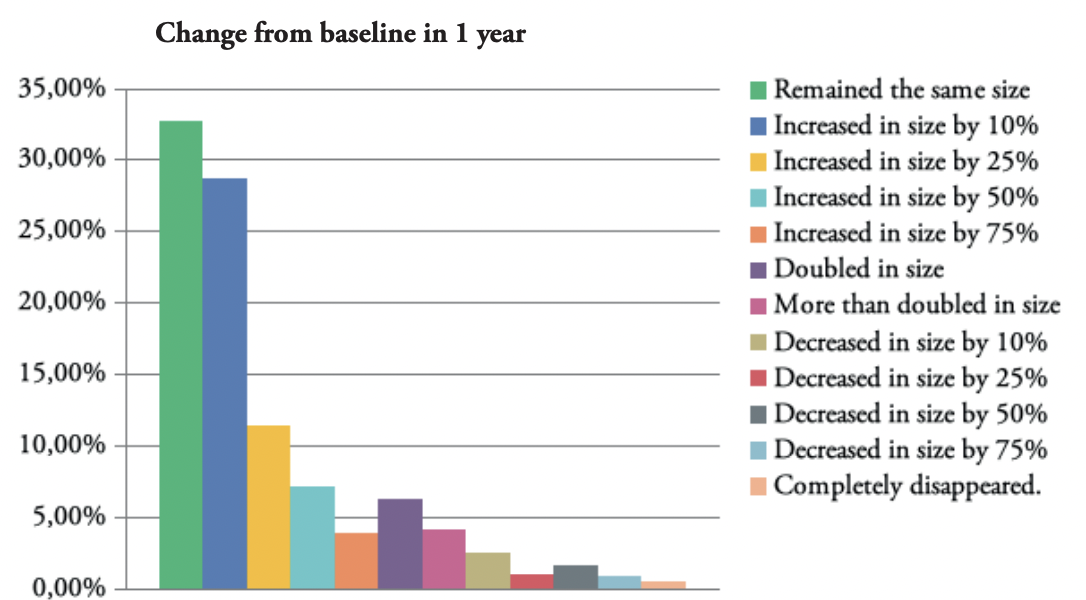
FIGURE 6: Graphic depiction of the change in the size of keloid lesions in 1 year.
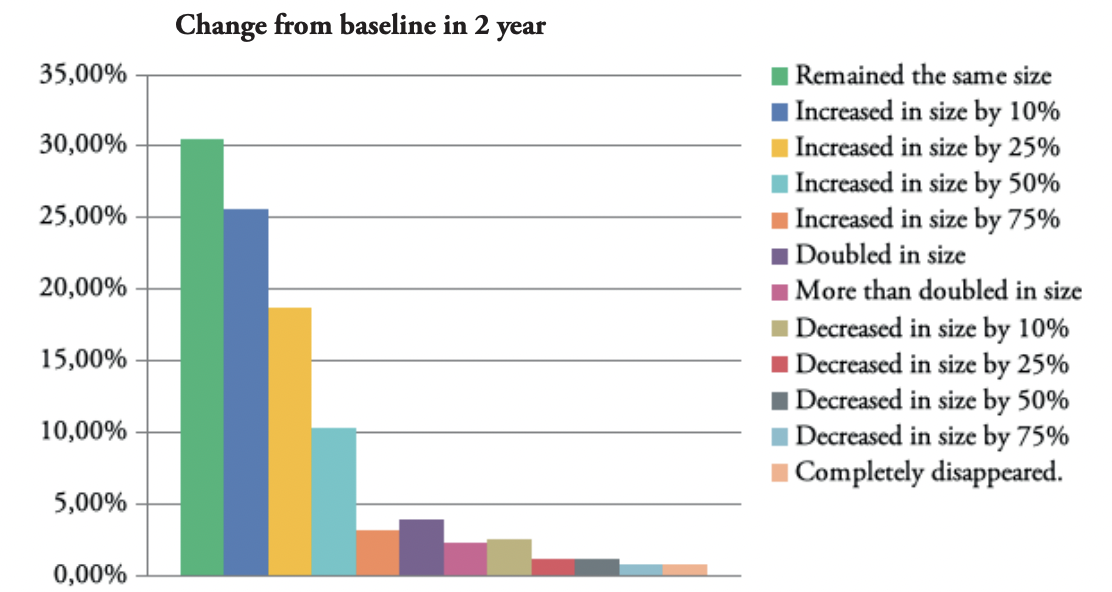
FIGURE 7: Graphic depiction of the change in the size of keloid lesions in 2 years.
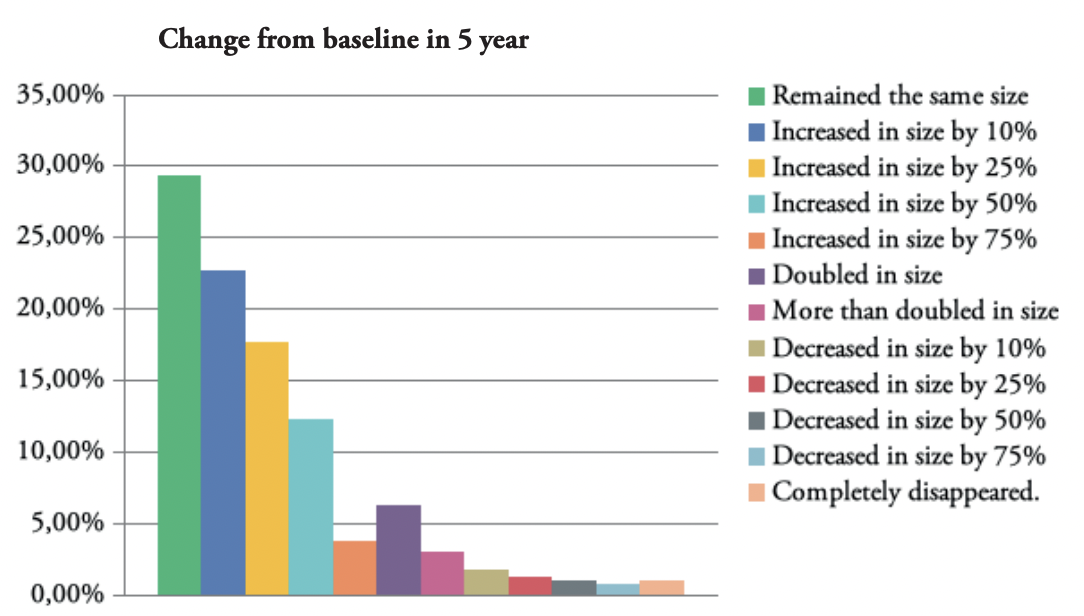
FIGURE 8: Graphic depiction of the change in the size of keloid lesions in 5 years.
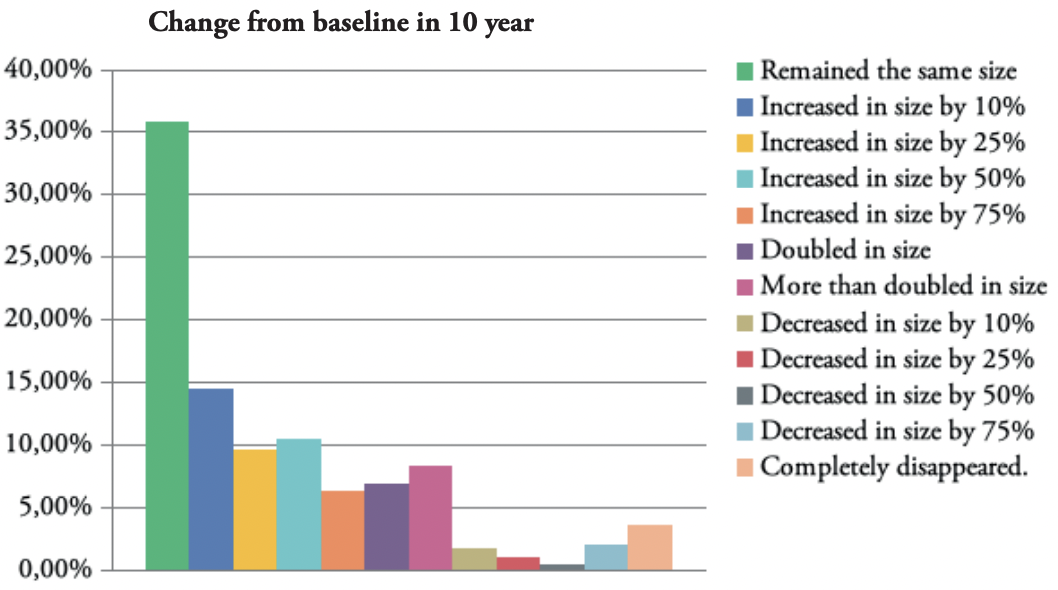
FIGURE 9: Graphic depiction of the change in the size of keloid lesions in 10 years.
Approximately, one third of the patients reported having stable disease and no progression of their KD at 1-, 2-, 5-, and 10-year timepoints. About 10% of the patients reported a 50% increase, and approximately 6% reported doubling of their keloids’ sizes at 1-, 2-, 5-, and 10-year timepoints. Approximately 6% of the patients reported some degree of reduction in their keloids’ sizes at 1-, 2-, and 5-year timepoints and about 8% reported some reduction at a 10-year timepoint.
DISCUSSION
Conducting clinical and basic science research on KD is a fundamental step in understanding this understudied disorder. However, when research is conducted on an unselected or an undefined patient population, the captured data cannot be properly analyzed and interpreted. The Cartesian tables introduced here using the clinical dataset from 971 keloid patients from the first author’s study, 976 patients from the co-author’s analysis, and the survey dataset from 1,709 participants clearly show the presence of different subsets of keloid patients, each with different biological and clinical behaviors. In conducting randomized trials on any disease condition, comparison of clinical or laboratory findings is permissible only within homogeneous patient populations, especially in studies that enroll a small number of patients, and KD is no exception to this rule
Each dataset provided a unique window into the natural history of KD and its growth rate over time among very large and diverse cohorts of patients. The clinical dataset was investigated by grouping patients by KD duration. The survey dataset asked participants to compare their keloids’ size to what they might have been at four separate timepoints in the past. Dataset analysis confirmed that in some KD patients, despite the passage of time, the disease had not progressed. This outcome was seen in 78 clinical dataset patients (8.02%) who remained in stage I for ≥15 years, and 35% of survey dataset patients for whom the disease had not progressed in 10 years.
Furthermore, KD can rapidly evolve into stage IV as observed in four clinical dataset patients who developed stage IV disease in ≤3 years. The same analysis showed that 15 (1.54%) patients developed stage III disease in ≤3years. Data from the survey dataset confirmed the same finding, as shown by the rate of doubling in the size of keloid lesions over time. This analysis showed that the disease doubled in size in 1 year in 84 (6.19%) patients and more than doubled in size in 1 year in 55 (4.06%) patients.
As shown in Figures 6–9, most patients experienced progression instead of reduction in their keloids’ size. In total, only 6%–8% of the patients reported some degree of reduction in keloid size, perhaps as a result of treatment. This fact is a distressing testament to the lack of effective treatments for most patients with KD.
LIMITATIONS
This study had several limitations. The measurements of the keloid lesion’ sizes were not accurate since a scale was not used while photographing the lesions. The authors used their best judgment to retrospectively estimate the size of lesions from photographs that lacked objective scale. Additionally, surveys are known to have their own limitations. The keloid survey was completed by individuals whom we believe had keloids. The authors had no contact with any survey participants and could not verify the accuracy of the information provided by them.
The authors proposed and used a staging system that has not been used or validated in the past. Indeed, this study marks the first time that Tirgan’s Keloid Staging System has been used in a large-scale assessment of keloid patients. The staging system was adopted from the TNM tumor staging system for solid tumors. We hope that in the future, others will be able to adopt, revise, or validate our staging system or propose better methodologies. Validating a staging system is conducted by comparing the effectiveness of systemic treatments and assessment of long-term outcomes of diseases [8].
Finally, the clinical dataset was biased because it was limited to the patients seen by the first author in his keloid specialty medical practice. The authors are cognizant that this patient cohort is not a true representation of all keloid patient populations. The data obtained from the online survey was also biased toward those searching the internet for information related to their illness or subjects exploring treatment options for their illness, with possible overrepresentation of younger and more computer-literate individuals. Since the survey was administered in English, it likely excluded non-English-speaking keloid patients.
CONCLUSIONS
Despite these limitations, the study found clinically relevant and applicable facts showing the variable clinical behaviors and rates of growth of KD among different patient subgroups. These findings have important implications for the design of clinical and laboratory research. To the authors’ knowledge, this is the first systematic and largescale review of the clinical behaviors of KD and the largest study ever conducted on this disease entity.
Armed with this knowledge, we shall no longer plan for or accept the results of small, single-arm or even randomized studies in KD without knowing which patient subgroups were enrolled. Furthermore, our results indicate that it will be unacceptable to conduct laboratory research on a limited number of keloid cell lines without knowing the clinical behavior of the underlying disease and origin of the keloid cells because conclusions drawn from any such studies will simply be incorrect and misleading.
CALL FOR ESTABLISHMENT OF AN INTERNATIONAL KELOID REGISTRY
The analysis results of the two large datasets in this study provide an opportunity to better understand the natural history and variable clinical behaviors of KD among different patient subpopulations. Further analysis of potential differences in KD clinical behaviors by using other variables will require a much larger database. Exploring the roles of other variables (such as age, ethnicity, gender, triggering factors, surgical interventions, and radiation) in the natural history and clinical behaviors of KD will require access to much larger datasets.
The precedence for such an approach has been established by several disease-specific registries. Patient registries are organized methodologies for collecting uniform data for particular indications over time. Registries play an integral role in improving our understanding of and answering numerous questions related to the disease being followed. For example, cancer registries, the oldest registries in the United States, have provided valuable information to the cancer research community.
Establishing an international keloid registry will enable data collection from a large number of keloid patients worldwide. The ideal registry would allow patients and clinicians to register and input data into the database in a secure and HIPAA-compliant manner. All patientrelated information must be de-identified. Each patient should be assigned an identification number, which he or she can then share with the treating physicians who can then input all relevant information into the registry. Those accessing the registry data should not have access to any patient-identifiable information.
Conflict of interest disclosure
The authors have no conflicts of interest to disclose.
ORCID
Michael Tirgan https://orcid.org/0000-0002-4761-403X
Eva Kerby https://orcid.org/0000-0003-4705-528X
References
- Saddawi-Konefka R, Watson D. Nonsurgical treatment of keloids and hypertrophic scars. Facial Plast Surg. 2019;35(3):260-266. doi: 10.1055/s-0039-1688847. Epub 2019 Jun 12. [PubMed] [CrossRef] [Google Scholar]
- Tirgan, MH. Intra-lesional steroid injections: Can they be harmful to some patients? Results of an online survey. Int J Keloid Res. 2017;1(1):21-28. [CrossRef ] [Goog le Scholar]
- Tirgan, M. Massive ear keloids: Natural history, evaluation of risk factors and recommendation for preventive measures—A retrospective case series. F1000Research 2016;5:2517. [PubMed] [CrossRef] [Google Scholar]
- Tirgan, MH. Neck keloids: Evaluation of risk factors and recommendations for keloid staging system [version 1; referees: 1 approved with reservations] 2016;5:1528. [PMC free article] [PubMed] [Google Scholar]
- Tirgan, MD. Lasers in treating keloid lesions: Can the treatment be harmful to some patients? Results of an Online Survey. Keloid Research. 2019;3(1):152-160. [CrossRef]
- Smith OJ, McGrouther DA. The natural history and spontaneous resolution of keloid scars. J Plast Reconstr Aesthet Surg. 2014;67(1):87-92. doi: 10.1016/j.bjps.2013.10.014. [PubMed] [CrossRef] [Google Scholar]
- Lu W-s, Zheng X-d, Yao X-h, Zhang L-f. Clinical and epidemiological analysis of keloids in Chinese patients. Arch Dermatol Res. 2015;307:109-114. 10.1007/s00403-014-1507-1. [PubMed] [CrossRef] [Google Scholar]
- Zhang G, Li R, Zhao X, Meng S, Ye J, Zhao L. Validation of the American Joint Committee on Cancer eighth edition staging system in patients undergoing hepatectomy for hepatocellular carcinoma: a US population-based study. J Surg Res. 2018;222:55-68. doi: 10.1016/j.jss.2017.09.044. Epub 2017 Nov 1. PMID: 29273376. [PubMed] [CrossRef] [Google Scholar]
