Can keloid lesions be ascribed? examining the relationship between keloid disorder, perCeived psyChosoCial distress, and serum neuropeptide y
Alison T. Tran, M.A., Ed.M,
Richard S. Feinn, PhD, Lynn C. Copes, PhD
ABSTRACT
Background: The relationship between the mind and skin has long been hypothesized to exist as the brain and skin share an embryologic ectodermal origin and are affected by similar neuro-hormonal factors. Neuropeptide Y (NPY) is often regarded as a stress-mediated “resilience” peptide. As a pleiotropic factor, NPY exerts diverse effects across many systems. Exogenous and endogenous stress may induce NPY release, which may lead to enhanced immune response via lymphocytic proliferation. In turn, both lymphocytes and macrophages produce NPY once activated. Scar formation is regulated by macrophages; specifically, the M2 phenotype regulates the repair process and scar outcome through the secretion of growth factors, which encourages proliferation, angiogenesis, and fibroblast differentiation into collagen secreting myofibroblast cells. However, excess collagen may lead to fibrosis. In this study, we focus on three of NPY’s roles: the mediation of stress, the promotion of angiogenesis, and its impact on the skin.
Objective: To investigate the relationship between keloid disorder, psychosocial distress associated with ascribed status (social status assigned at birth), and NPY, specifically its role in stress mediation, angiogenesis, and wound repair.
Method: A total of 37 (15 men, 22 women) Black, Hispanic, Asian, and White volunteers with and without keloids participated in this current study. The risk group consisted of keloid formers (8 males, 12 females) and the control group consisted of non-keloid formers (7 males, 10 females). During intake, participants were asked to provide information about their current health, income, and experience with trauma/ discrimination as well as complete a 22-item Psychological General Well-Being Index (PGWBI) and a 10-item Dermatology Life Quality Index (DLQI). Following this, participants had 2 ml of blood drawn via venipuncture before and after watching a stress-inducing video. NPY levels pre/post video were analyzed using commercially available sandwich ELISA kits.
Results: Preliminary results show a potential interaction between gender, race, and keloid status, in which Black men with keloid disorder showed a more exaggerated response (increased NPY levels) to acute stress (stereotype threat video) compared to Black women with keloid disorder. Moreover, there was a positive correlation between quality of life/stress and keloid status such that subjects with keloids have a higher DLQI than subjects without keloids (means of 4.40 vs. 2.65, p =. 040). Further analysis indicated a near significant difference between all four groups (male/no keloid, male/keloid, female/no keloid, female/keloid) and self-reported overall health and PGWBI.
Conclusion: As anticipated, Black men with keloid disorder showed a more robust response to acute stress, and overall, participants with keloid disorder reported more distress and lower quality of life associated with having keloid lesions. To our knowledge, no research has been done on the possible relationship between NPY, stress, and keloid disorder. Future research should use stricter inclusion criteria to control for confounds in order to determine true significance of preliminary results.
Key Points: NPY has been shown to modulate cutaneous and/or immune function. Preliminary results show a potential interaction between keloid disorder and gender that is mediated by NPY levels. This project aims to raise awareness on the impact of psychosocial conditions on symptomology as well as highlight the importance of coordinating psychological services within dermatological care for keloids.
INTRODUCTION
A keloid lesion is a fibrous tissue overgrowth of the skin characterized by benign hyperproliferative growth of dense fibrous tissue caused by the invasive growth of keloid fibroblasts, a class of ‘activated’ fibroblasts that stimulate proliferation and abnormal deposition of extracellular matrix.1 Keloid lesions can be painful, pruritic, and are generally more common in darker pigmented skin;2 specifically, African American, Latin American,1 and Asian American2 individuals. Moreover, between 4% and 16% of darker pigmented individuals report decreased physical and/or mental health due to pain and disfigurement accompanying keloids.1 Keloid pathogenesis is inadequately understood, but it has been associated with genetic and environmental factors.3,4
Manifestation of illness can extend beyond race and attributed to epigenetic modification. Racial inequalities in health and healthcare have been extensively researched and remain a long-standing topic of academic debate.5,6,7 Self-reported discrimination at the individual level has been linked to a wide range of health issues such as elevated blood pressure, breast cancer, preterm delivery/low birth weight, and depression.6 Krieger proposed the construct of embodiment to describe how social inequalities shape the biology of racialized groups as “no aspect of our biology can be understood absent knowledge of history and individual and societal ways of living.”8 Gravlee6 argues that racial inequalities “become embodied” in the biological well-being of racialized groups/ individuals as the sociocultural reality of race and racism has biological consequences for racially defined groups.
The prevalence of psychiatric co-morbidity among dermatology patients is approximately 30-40%, which is slightly higher than that of neurological, oncological, and cardiac patients combined.9 The relationship between the mind and skin has long been hypothesized to exist as the brain and skin have a common embryological ectodermal origin and are affected by similar neuro-hormonal factors.9
Neuropeptide Y (NPY) as potential biomarker in keloid disorder
NPY is a highly conserved 36-amino acid neuropeptide abundantly expressed in both the central and peripheral nervous system (CNS and PNS). As a pleiotropic factor, NPY exerts diverse effects across many systems, e.g., cardiovascular, neuronal and immune, with distinct actions centrally and peripherally.
In the CNS, NPY is anxiolytic and anti-epileptic, inhibits sympathetic activity, and is hypotensive.10 Centrally, increased NPY is associated with physiological resilience, memory improvement, neurogenesis, and increased performance during military training.11 NPY enhances stress coping abilities11 and resilience to stress12 as it is abundantly expressed in brain regions known for stress and emotional regulation.13 Peripherally, NPY amplifies the stress response and induces vasoconstriction through potentiation of catecholamines through its Y1 receptor.11 There is not only mounting evidence for the role of NPY as a mediator of centrally mediated stress resilience,12 but also in the peripheral mediation of chronic stress due to its effects on the immune system and angiogenesis. NPY-dependent angiogenesis plays a role in wound healing, vascularization in ischemia, aging, and induces basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) expression.10
In a recent study by Suarez et al., NPY along with 20 other biomarkers was screened via real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) and In-Cell Western blotting in tissue biopsies and primary fibroblasts. 14 The NPY gene expression via extracted total RNA from tissue biopsies of normal skin, keloid skin, and hypertrophic skin enabled the analysis of mRNA expression at the tissue and cellular levels. mRNA levels were found to be down-regulated for NPY biomarkers in keloid tissue biopsies, but PCR analysis of NPY gene expression from primary fibroblasts showed significantly higher expression of NPY in keloid tissue (p < 0.5).14 Though the mismatch between tissue mRNA and cellular mRNA NPY expression may be surprising, moderate and varied correlations illustrate that mRNA expression is not entirely and perfectly predictive for protein expression.15 Further, NPY protein expression in primary fibroblast cultures by In-Cell Western blotting showed comparable expression between normal skin and NPY fibroblasts, but significantly less expression in keloid fibroblasts compared with hypertrophic fibroblasts (p < 0.05). The lack of expression makes sense as NPY is preferentially secreted during times of intense stress,10 which was not a condition examined.
This current pilot study examines NPY as a potential stress indicator in blood specifically in relation to the development of keloid disorder in the setting of psychosocial distress. Methods
This student capstone study was conducted with the approval of the Institutional Review Board of Frank H. Netter MD School of Medicine at Quinnipiac University, protocol #04518.
Participants
A total of 37 (15 men, 22 women) Black, Hispanic, Asian, and White volunteers with and without keloids participated. The risk group consisted of keloid formers (8 males, 12 females) and the control group consisted of non-keloid formers (7 males, 10 females).
Inclusion and exclusion criteria
Criteria for selection included volunteers 18 years of age or older, self-identified as Asian, Black, and/or Black Hispanic/Latino, with a history of keloid disorder.
Quality of life questionnaires
During the intake, participants were asked to provide information about their current overall health and experience with trauma/ discrimination. They were also asked to complete a 22-item PGWBI measuring six subscales (anxiety, depressed mood, positive well-being, self-control, general health, and vitality) and a 10-item DLQI. Participants had 2 ml of blood drawn via venipuncture before and after watching a stress-inducing, 5-minute clip that demonstrated a case of stereotype threat involving a police encounter as individuals who experience stereotype threat cope with anxiety and engage in self-regulatory behaviors such as vigilance to threat-related cues. Subjects were informed of the option to withdraw from the study at any point and were debriefed shortly after watching the video due to its possible distressing content.
ELISA
A commercially available Human NPY Sandwich ELISA kit from Millipore (Cat. #EZHNPY-25K) was used to test the blood samples for NPY levels. The limit of sensitivity of this assay was 2 pg/mL NPY in a 50 μL sample size. Enzyme activity was measured spectrophotometrically at 450 nm and 590 nm.
Statistical analysis
For statistical analysis, a Paired Samples t-test was used to compare changes in NPY before and after stress inducing video. The independent samples test (2-tailed) was used to compare self-reported overall health, PGWBI, and DLQI responses in control versus risk groups. The univariate general linear model was used to test if stress exposure affected NPY levels differently by ethnicity and gender when ethnicity (Black vs other) and gender were between subjects’ factors and time point (pre versus post stress video) was a within subjects factor. In the analyses we report the test statistic (t for t-tests and F for general linear model), p-value, and standardized effect size Cohen’s d. Effect sizes of 0.2, 0.5, and 0.8 or greater were considered small, medium, and large, respectively.16 All analyses were conducted in SPSS v25 and the alpha significance level was set at 0.05.
RESULTS
The participants’ demographic characteristics are shown in Table 1. Participants with keloids were similar to participants without keloids on all demographic variables. Most participants were under 30 years of age, had a BMI level above the normal range (19-25), and an income under $50,000.
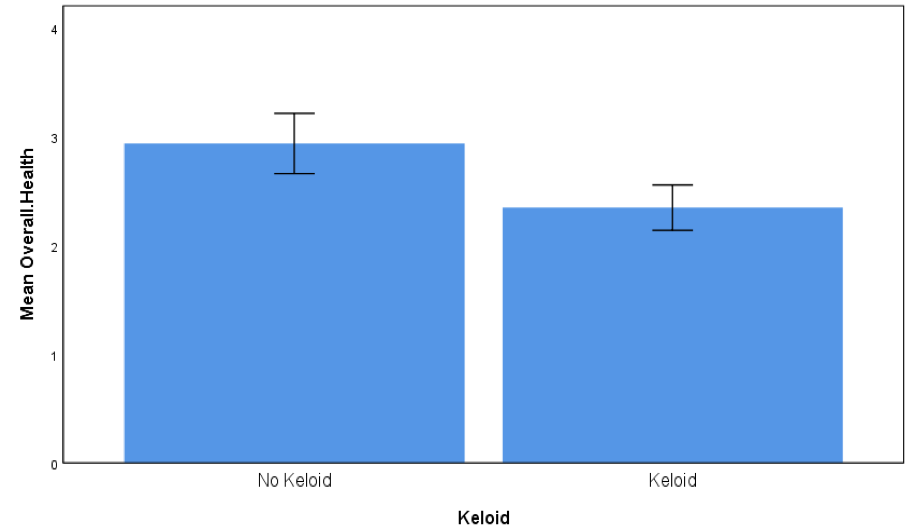
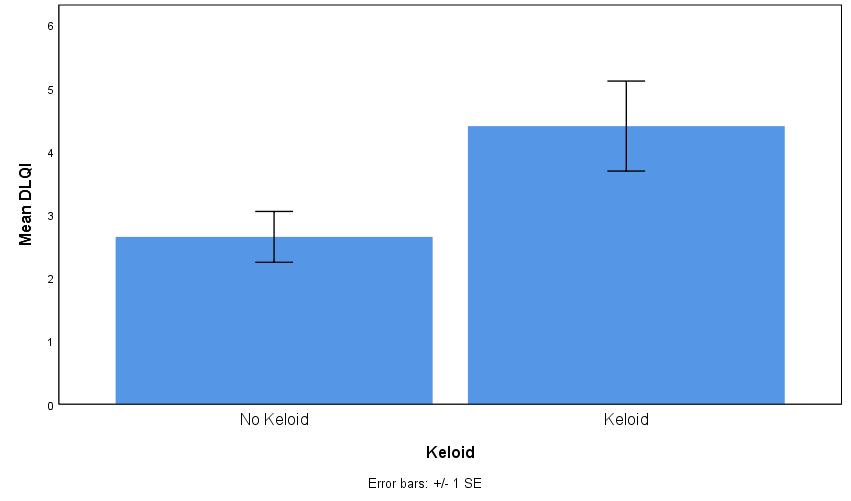
Participants with keloids compared with participants without keloids reported lower overall health (t = 1.73, p = 0.09, d = 0.57, Figure 1) and scored significantly higher in the DLQI (t = 2.14, p = 0.040, d = 0.72, Figure 2), where higher DLQI scores signify a larger effect on the patient’s life. Scores of participants with keloids were similar to those without keloids in the PGWBI (t = 0.88, p = 0.39, d = 0.30, Figure 3).
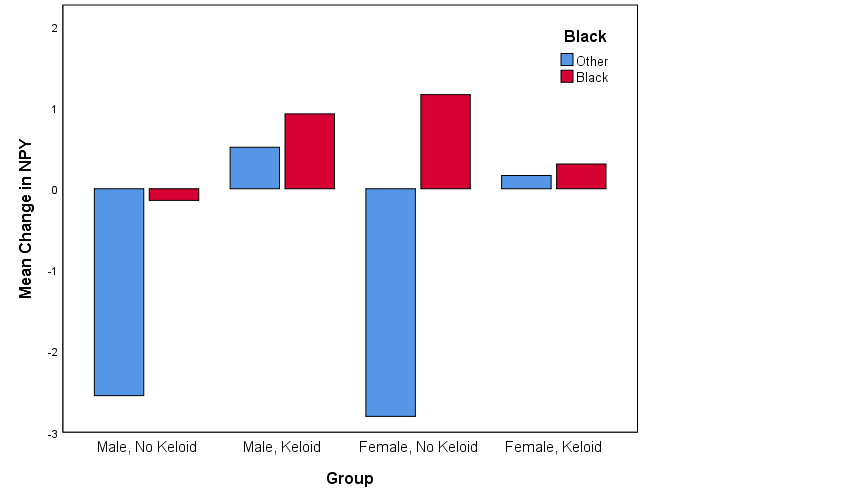
Overall, across all participants there was not a significant change in NPY levels from pre to post stress (t = 0.09, d = 0.02, p = 0.93). Nor was there a significant difference between keloid status from pre to post stress tests (F = 1.32, d = 0.38, p = 0.26). When including race and gender in the model there were no statistically significant interactions. However, there appeared to be possible interaction effects as shown in Figure 4. Black participants showed a tendency for greater change in NPY after viewing the video and Black males with keloid disorder tended to show more change than Black females with keloid disorder.
Table 1: Role of ethnic skin type in response to treatment and potential to worsen keloid
| Demographics | No Keloid (n=17) | Keloid (n=20) | P-Value |
| Gender Female Male | 10 (59%) 7 (41) | 12 (60%) 8 (40) | .942 |
| Race Black Other | 11 (65%) 6 (35) | 14 (70%) 6 (30) | .732 |
| Age 18-30 31-40 Over 40 | 9 (53%) 4 (24) 4 (24) | 9 (45%) 7 (35) 4 (20) | .748 |
| BMI Mean ± SD | 33.8 ± 10.9 | 30.2 ± 9.6 | .293 |
| Income < $10,000 $10,000-49,999 $50,000 + | 5 (29%) 9 (53) 3 (18) | 6 (30%) 6 (30) 8 (40) | .254 |

DISCUSSION
Immune function and angiogenesis
NPY secreted from nerve fibers in skin can modulate cutaneous and/or immune cell function.17 In a positive feedback fashion, exogenous and endogenous stress may induce the release of NPY, which may lead to enhanced immune response via lymphocytic proliferation;17 in turn, both lymphocytes and macrophages produce NPY once activated.18 Macrophages regulate wound repair and are responsible for re-vascularization and wound re-epithelization mediated through their pro-inflammatory M1 phenotype and anti-inflammatory M2 phenotypes.19 The M2 macrophage phenotype specifically regulates the repair process and scar outcome through the secretion of growth factors, which not only encourages fibroblast proliferation/differentiation into collagen secreting myofibroblasts, but also angiogenesis.19 NPY has also been shown to mediate growth and angiogenesis in vivo20,10 mainly through its Y2 and Y5 receptors.10
NPY interacts with the HPA and SAS axis
The stress response is primarily mediated by two groups of hormones: glucocorticoids (cortisol) and catecholamines (norepinephrine and epinephrine). These hormones are governed by the hypothalamic-pituitary-adrenal axis (HPA) and the sympatho-adrenomedullary system (SAS), respectively. NPY interacts with the HPA axis in both the CNS and PNS in a reciprocal manner of regulation. It has been suggested that increases in NPY within the hypothalamus are implicated in the initial activation of the HPA axis resulting in the release of cortisol releasing hormone (CRH) from the paraventricular nucleus (PVN), and reciprocally, glucocorticoids may upregulate mRNA NPY expression in the arcuate nucleus, which further strengthens the stress response.11 NPY, norepinephrine (NE), and ATP are co-transmitters within the sympathetic nerves, but demonstrate differential release; NE is preferentially released during acute stress and for tonic sympathetic activation, while NPY is favorably released during prolonged and/or intense stress.10 NPY mediates the effects of both ATP and NE in several ways. Post-synaptically, NPY causes direct vasoconstriction by activating its Y1 receptors in various regions of the cardiovascular system and by indirect potentiation of the vasomotor contractile functions of ATP and NE.11 Pre-synaptically, NPY reduces sympathetic activation by dampening the release of NE, ATP, and itself through its Y2 receptors.10 NPY’s pre-synaptic Y1, Y2, and Y5 receptors were also found to inhibit catecholamine synthesis.21
Chronic stress regulator
Stressors elicit responses that enable an organism to adapt to changing or threatening environments. However, prolonged and/or severe stress can lead to maladaptive responses within the stress circuitry and can manifest as stress-related psychiatric diseases.12 Research has underscored the effects of neuromodulators on stress-related emotionality. Specifically, NPY has been implicated as a stress regulatory transmitter in PTSD22 and has been linked to mood altering disorders as reduced CNS NPY levels are associated with anxiety, depression, and PTSD in humans.13 In PTSD, CNS NPY counteracts the activity of pro-stress NE and anxiogenic cortisol releasing hormone (CRH).22 In addition to decreased CSF NPY concentration in combat veterans with PTSD,22,13 baseline plasma NPY were found to be reduced in volunteers with PTSD compared to those without.23 Further, NPY may play a role in resilience as Yehuda and colleagues found significantly higher plasma NPY levels in recovered individuals with a prior history of PTSD compared to those without a prior history.24
NPY, stress, and skin
Extensive research has been conducted on the effects of psychosocial stress/discrimination and hormones on the physical body. Chronic experiences of stress may lead to adverse health outcomes in the form of “stress-related disease,” a term first coined by Hans Seyle.11 In an obesity study conducted by Kim et al., NPY was found to play a role in the pathophysiologic relationship between stress and obesity, although stress itself did not cause obesity, which highlights the importance of “NPY-by-stress interaction studies.” 25 Glucocorticoids released during stressful times stimulate NPY release, which leads to increased appetite, feeding behaviors, and subsequent fat gain.25 No significant single nucleotide polymorphisms (SNPs) were connected to obesity when SNPs were examined without consideration of psychosocial stress. However, NPY SNPs were significantly associated with obesity phenotypes such as BMI, waist circumference, and visceral adipose tissue (VAT) when psychosocial stress levels were considered. Further, a role for NPY in the pathogenesis of vitiligo has been proposed after elevated NPY levels were found in skin depigmented by vitiligo26,17 and in the plasma of individuals with vitiligo,27 indicating a possible relationship between NPY, lymphocytic proliferation, and subsequent melanocytic destruction.17
Current study
Evidence for racial inequalities in morbidity and mortality across multiple biological systems is well documented.7 At the institutional level, racism affects access to care, differential quality of care, and health outcomes.28 Considering racism’s effects while recognizing that keloid lesions are more prevalent in darker pigmented skin, an investigation of NPY’s potential impact on keloid lesion and its ability to serve as a marker of stress prompted this study.
Our hypothesis of a race and gender effect in stress response via increased NPY (and in keloid-prone individuals) was inspired by previous research showing evidence for gender- and race-related differences in response to police-related stereotype threats, in which Black men experienced the most stereotype threat measured in anticipated anxiety and self-regulatory efforts such as the effort to avoid “suspicious” behavior.29 Our finding of a potential gender and race interaction effect with Black participants showing more change in NPY and Black males with keloid disorder showing more change than Black females with keloid disorder may be clearer if we were able to statistically control for differences in medication therapy (immunosuppressant), history of surgical removal and/or various therapies (injection, cryotherapy, etc.), and BMI (increased expression of NPY in adipose tissue).
The relationship between cutaneous disorders and decreased quality of life is supported by many psychodermatology studies.30, 31, 32 As expected, and in line with extant literature, those with keloid disorder scored significantly higher on the DLQI index, indicating more distress and lower quality of life associated with having keloid lesions.33 Keloid lesions can be pruritic, painful, and enhance over time. Depending on the location of the keloid lesions, it may be conspicuous and may lead to lower self-esteem/confidence and social isolation. Moreover, participants with keloids, compared to participants without, reported lower overall health, which may be attributed to having a chronic inflammatory skin condition. There is also evidence for the negative correlation between inflammatory conditions and quality of life, with some studies showing a connection to depression.34
On the contrary, there was no significant difference found for overall psychological well-being measured by the PGWBI scale, perhaps because it is a general well-being index rather than a dermatologic-specific one. Besides measuring subjective well-being, the scale purports to measure subjective distress stemming from affective/emotional states (anxiety and depressed mood). However, the DLQI more specifically examines quality of life as it relates to cutaneous disorder. It is possible that participants with keloids report lower quality of life, but do not necessarily experience clinical anxiety and/or depression. Further, barriers such as mental health stigma, exacerbated by cultural and cognitive views, has been shown to be more prominent in ethnic/racial minority populations.35 These barriers may lead to denial, avoidance/reluctance, or downplay of severity as well as underutilization of mental health services.
By 2044, the “minority” races will together become the majority in the United States.36 Thus, it is imperative not only to understand and recognize how dermatologic conditions manifest in skin of color, but also to consider psychosocial factors in patient symptomatology given the extent of psychiatric comorbidity in dermatologic settings. Further, we encourage collaboration amongst providers to include psychiatry, psychology, mental health, and social workers in order to promote longevity of care to dermatologic patients who may need long-term mental health surveillance. This collaboration will also allow for continuity of care by ensuring smooth transitional care.
Limitations
One limitation of this current study is the small sample size, which is partly due to limited student capstone budget. Another limitation is the lack of strict inclusion criteria to include only those with current keloid lesions, not on medication management, and with no history of surgical removal of lesions in order to truly examine the impact of NPY stress-mediated release on keloid disorder. Caution is needed regarding the generalizability of findings as the potential relationship examined is stress associated with ascribed status. Though discrimination based on ascribed status is objective behavior, perceived distress associated with the perception of discrimination is subjective and is contingent upon the individual interpretation and internalization of such events. Several factors that may contribute to stress level of our volunteers include: age at presentation, age of onset of keloid disorder, extent of keloid involvement of the skin (i.e., staging of the disease), presence or absence of clinical symptoms from keloid (pain, pruritus), success or failure of prior treatments, location of keloid lesions, family history of keloids (anticipation of developing keloids in a young person born to parents with keloids), and financial status of the patient, which was collected as part of demographic intake survey. These factors should be incorporated into future studies.
Future Directions
To our knowledge, no research has been performed on the possible relationship between NPY and stress in keloid disorder. Future research should use stricter inclusion criteria to control for confounds in order to determine true significance of preliminary results. As such, future research should consider replicating this study by examining stress induced NPY with a more refined sample. Another alternative study, one which is currently underway, is to assess for co-released substances that accompany NPY in the PNS such as cortisol. The potential for the examination of other biomarkers associated with keloid disorder such as TGF- in the setting of NPY release should be considered. As for social advocacy, there is potential to lobby on behalf of keloid formers who cannot afford insurance coverage if there is explicit evidence for the role of NPY-mediated stress in keloid formation.
Acknowledgements
We acknowledge Emily Pagan, Joseph B. Watson, and Gabbriel Ceccolini, MS for their assistance with this study.
Citations
- Xin Y, Wang X, Zhu M, Qu M, Melia B, Lin L, …Zhang Y. Expansion of CD26 positive
fibroblast population promotes keloid progression. Experimental Cell Research. 2017;356(1):104-113. https://doi.org/10.1016/j.yexcr.2017.04.021
- Butler PD, Ly DP, Longaker MT, Yang GP. Use of organotyping co-
culture to study keloid biology. Am J Surg. 2009;195(2):144-148. http://doi.org/10.1016/j.amjsurg.2007.10.003
- Jfri A, Rajeh N, Karkashan E. A case of multiple spontaneous keloid scars. Case
Reports in Dermatology. 2015;7(2):156–160. http://doi.org/10.1159/000437249
- Robles DT, Moore E, Draznin M, Berg D. Keloids: Pathophysiology and management.
Dermatology Online Journal. 2007;13(3):9
- Keppel KG, Pearcy JN, Wagener DK. Trends in racial and ethnic-specific rates for the
Health Status Indicators: United States, 1990-1998. Healthy People 2000 Stat Notes. 2002;23:1-16
- Gravlee CC. How race becomes biology: Embodiment of social inequality. J. Phys.
Anthropol. 2009;139:47–57. http://doi.org/10.1002/ajpa.20983
- Oliver MN. Racial health inequalities in the USA: The role of social class. Public Health.
2008;122(12):1440-1142. http://doi.org/10.1016/j.puhe.2008.05.014
- Krieger N. Embodiment: A conceptual glossary for epidemiology. J Epidemiol
Community Healt. 2005;59:350–355. http://doi.org/doi:10.1136/jech.2004.024562
- Ghosh S, Behere RV, Sharma P, Sreejayan K. Psychiatric Evaluation in Dermatology:
An Overview. Indian Journal of Dermatology. 2013;58(1):39–43. http://doi.org/10.4103/0019-5154.105286
- Kuo LE, Zukowska Z. Stress, NPY and vascular remodeling: Implications for stress-
related diseases. Peptides, 2007;28(2):435–440. http://doi.org/10.1016/j.peptides.2006.08.035
- Hirsch D, Zukowska Z. NPY and Stress 30 Years Later: The peripheral view. Cellular
and Molecular Neurobiology, 2012;32(5):645–659. http://doi.org/10.1007/s10571-011-9793-z
- Enman NM, Sabban EL, McGonigle P, Van Bockstaele EJ. Targeting the Neuropeptide
Y system in stress-related psychiatric disorders. Neurobiology of Stress. 2015;1:33–43. http://doi.org/10.1016/j.ynstr.2014.09.007
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, Geracioti TD. Low
cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66(7):705–707. http://doi.org/10.1016/j.biopsych.2009.04.037
- Suarez E, Syed F, Alonso-Rasgado T, Bayat A. Identification of biomarkers involved in
differential profiling of hypertrophic and keloid scars versus normal skin. Arch Dermatol Res. 2015;307(2): 115-133. https://doi.org/10.1007/s00403-014-1512-4
- Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H,
Tan L, Xie J, Zhu X, Liang S, Deng H. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai). 2008;40(5):426-236. https://doi.org/10.1111/j.1745-7270.2008.00418.x
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY:
Routledge Academic; 1988
- Lazarova R, Hristakieva E, Lazarov N, Shani J. Vitiligo-related neuropeptides in nerve
fibers of the skin. Archives of Physiology and Biochemistry. 2008;108(3):262-267. http://doi.org/10.1076/1381345520000710831ZFT262
- Stanojevic S, Mitić K, Vujić V, Kovacević-Jovanović V, Dimitrijević, M. Exposure to
acute physical and psychological stress alters the response of rat macrophages to corticosterone, neuropeptide Y and beta-endorphin. The International Journal on the Biology of Stress. 2007;10:65-73. http://doi.org/10.1080/10253890601181289
- Hesketh M, Sahin KB, West ZE, Murray, RZ. Macrophage phenotypes regulate scar
formation and chronic wound healing. International Journal of Molecular Sciences, 2017;18(7):1545. http://doi.org/doi:10.3390/ijms18071545
- Salo P, Bray B, Seerattan R, Reno C, McDougall J, Hart DA. Neuropeptides regulate
expression of matrix molecule, growth factor and inflammatory mediator mRNA in explants of normal and healing medial collateral ligament. Regulatory Peptides. 2007;142(1-2):1-6. https://doi.org/10.1016/j.regpep.2007.01.001
- Westfall TC. In: Prejunctional effects of neuropeptide Y and its role as a cotransmitter.
Michel MC, editor. Berlin: Springer; 2004. pp. 137–183
- Schmelter SN, Herman, JP, Sah R. Neuropeptide Y (NPY) and posttraumatic stress
disorder (PTSD): A translational update. Exp. Neuro. 2016;284(B):196-210. http://doi.org/10.1016/j.expneurol.2016.06.020
- Rasmusson AM, Hauger RL, Morgan III CA, Bremmer JD, Charney DS, Southwick SM.
Low baseline and yohimbine-stimulated plasma neuropeptide y (NPY) levels in combat-related PTSD. Soc of Bio Psychiatry. 2000;47:526-539. http://doi.org/10.1016/s0006-3223(99)00185-7
- Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat
exposed veterans: Relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2005;59:660-663.
http://doi.org/10.1016/j.biopsych.2005.08.027
- Kim HJ, Min HB, Min JY. Neuropeptide Y gene-by-psychosocial interaction effect is
associated with obesity in a Korean population. Psychoneuroendocrinology. 2016;69:10. https://doi.org/10.1016/j.psyneuen.2016.03.003
- Laddha NC, Dwivedi M, Mansuri MS, Singh M, Patel HH, Agarwal N, … Begum R.
(2014). Association of Neuropeptide Y (NPY), interleukin-1B (IL1B) genetic variants and correlation of IL1B transcript levels with vitiligo susceptibility. PLoS ONE. 2014;9(9):e107020. http://doi.org/10.1371/journal.pone.0107020
- Tu C, Zhao D, Lin X. Levels of Neuropeptide-Y in the plasma and skin tissue fluids of
patients with vitiligo. Journal of Dermatological Science. 2001;27(3):178-182. https://doi.org/10.1016/S0923-1811(01)00134-7
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence
and needed research. J Behav Med. 2009;32(1):20-47. http://doi.org/10.1007/s10865-008-9185-0
- Najdowski CJ, Bottoms BL, Goff PA. Stereotype threat and racial differences in citizen’s
experiences of police encounters. Law and Human Behavior. 2015;39(5):463-477.
2005;15:339-344. http://doi.org/10.1037/lhb0000140
- Hong J, Koo B, Koo J. The psychosocial and occupational impact of chronic skin disease.
Dermatologic Therapy. 2008;21:54-59. http://doi.org/10.1111/j.1529-8019.2008.00170.xh
- Deshpande H, Kavita MB, Tripathy TB, Chaturvedi A. Assessment of
quality of life in patients with skin disorders undergoing ayurvedic panchakarma (biopurification) as management. Journal of Evidence-Based Complementary & Alternative Medicine. 2016;21(3):215–220 https://doi.org/10.1177/2156587215615026
- Tuckman A. The potential psychological impact of skin conditions. Dermatology
and Therapy. 2017;7(1):53-57. http://doi.org/ 10.1007/s13555-016-0169-7
- Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid
and hypertrophic scarring. Arch Dermatol Res. 2006;297(10):433-438. https://www.doi.org/10.1007/s00403-006-0651-7
- Faugere M, Micoulaud-Franchi JA, Faget-Agius C, Lancon C, Cermolacce M, Richieri
- Quality of life is associated with chronic inflammation in depression: A cross-sectional study. J Affect Disord. 2018;227:494-497. https://www.doi.org/10.1016/j.jad.2017.11.061
- Leong FT, Kalibatseva Z. Cross-cultural barriers to mental health services in the
United States. Cerebrum: The Dana forum on brain science. 2011;5.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population:
2014 to 2060. Population estimates and projections: Current population reports. The Census Bureau Web Site. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf Accessed March 25, 2019.
