Proceedings of 2nd International Keloid Symposium, Rome, Italy, June 19-21, 2018
Michael H. Tirgan, MD
Setting the Scene: Basic Understanding of Keloid Disorder
CLINICAL PRESENTATION OF KELOID DISORDER:
A Retrospective Study of 1088 Patients and Recommendation for Keloid Staging System.
Michael H Tirgan MD
Keloid Research Foundation, New York, USA
Medical terminology is a universal scientific language used to accurately describe (as precisely possible) the human body, its components as well as the disease processes.
Correct terminology and proper classification of diseases is at the core of understanding human illnesses. The term “Keloid” was used in 1800 to describe this complex genetic disorder, however, it soon acquired ‘Scar” as a component which still remains in use in 2018.
The term “scar” is defined as “a mark remaining (as on the skin) after injured tissue has healed. Surgical scar and acne scar are correct examples of using this terminology. However, referring to an active pustular acne lesion, as an acne scar will be improper, so will be using the term ‘” scar” in referring to an inflamed surgical wound while it is healing.
Moriz Kaposi, while working at Vienna University in his 1876, in his Lehrbuch der_ Hautkrankheiten (Textbook of Skin Diseases) described “hypertrophische narbe” and also used the term “narbekeloid”, later translated into English as hypertrophic scar and keloid scar.
In dealing with a serious chronic genetic skin disorder that is poorly understood, one that can serious impact a person’s quality of life, using the term “scar” is not only improper use of the term, but most importantly is misleading to the point the most health insurance companies refuse to pay for medical care of the patients, as they consider “treatment of a scar” as a cosmetic procedure.
As for the condition commonly known as “hypertrophic scar”, once again, utilization of the term “scar” improper as we are dealing with an active inflammatory wound healing reaction that results in localized hypertrophy.
Furthermore, at the heart of these terminology, we have an underlying genetic disorder, for which there has never been a proper terminology.
To remedy this, author proposed the following terminology:
- The term “Keloid Disorder” to define the underlying genetic disorder,
- The term “Keloid Lesion” to refer to a particular keloid lesion
- The term “Hypertrophic Dermal Healing Reaction” or “Hyper-DHR” to replace hypertrophic scar.
Age of Onset of Keloid Disorder, Correlation between the Age at Onset and Clinical Manifestation of the Disorder; A Retrospective Multivariate Study of 1329 Patients.
Fernanda Oliveria Balbino, PhD;
Michael H. Tirgan, MD
INTRODUCTION
Keloid Disorder (KD) has a very diverse phenotype and presents itself for most part during childhood and teenage years. Age of onset of KD is not well described in the literature and has not been subject of any prior research. Based on clinical observations, various authors have reported KD to be commonly seen between the ages of 10 and 30 with no clear indication of the age of onset of this disorder.
MATERIAL AND METHODS
In the current IRB approved online survey study we inquired from 1695 keloid patients to recall the age of onset of their first keloid. 1329 patients were able to recall the age at which they developed their very first keloid. Several univariate and multivariate analysis and probabilistic models were carried out in order to understand the changes in the distribution of the age of onset of the disorder according to different phenotypes. Updated dataset analysis will be presented at the meeting.
RESULTS
Analysis of this data revealed that the age of onset of KD for great majority (approximately 82%) of patients is between 5 and 25, with 54.6 % of patients being diagnosed before age of 18; establishing KD as one of the most common chronic cutaneous childhood disorders. Additionally, subset analysis of our data revealed a strong correlation between the age of onset and pattern of distribution of KD with shoulder, upper arm, ear and earlobe keloids to present at a much younger age as opposed to keloids in other regions. Similar correlation was detected between chicken pox as the triggering factor for KD.
DISCUSSION
Studying age of onset of a particular disorder is among the most fundamental steps in understanding the illness. This knowledge allows us to devote proper resources, research or societal, where they are mostly needed. In this study, we analyzed the age of onset of keloid and its association with a set of covariates. To our knowledge, this is the first time that the age of onset of KD is being systematically studied and reported.
pediatric keloid disorder
PEDIATRIC KELOID DISORDERCase Series of 30 Patients from Tunisia
Bahloul E1, Mseddi M1, Bouchaala M1, Frikha F1, Boudaya S1, Masmoudi A1, Turki H1
Introduction: The aim of our study was to review the epidemiological and clinical characteristics of the keloid disorder in children focusing particularly on the therapeutic features through a hospital series.
Methods: We performed a retrospective study of all cases of childhood keloids diagnosed in our dermatology department between 2015 and 2018 (30 months). The inclusion criteria consisted of keloid lesions confirmed by clinical evaluation or histological examination in patients aged between 2 and 16 years old.
Results: Over 30 months, we collected 30 cases including 19 girls and 11 boys (Sex ratio F/ M = 1.72). The mean age was 10.7 years with an age superior to 10 years in 19 cases. The mean duration of the lesions varied between 18 months and 7 years with an average of 2.5 years. A personal history of keloids was noted in 2 cases. The triggering factors for development of keloids lesions were: burns in 11 cases (36.66%), injuries in 6 cases (20%), chickenpox scars in 6 cases (20%), surgery in 6 cases (20%) and a piercing in 1case (3%). keloids were solitary in 22 patients (73.3%) and multiple in the other 8 cases (26.6%). Localized itching was noted in 20 cases associated with pain in 14 cases. The shape of the lesions was linear in 19 cases (figure) and nodular in 11 cases. Lesions were located at 1 Department of Dermatology, Hedi Chaker Hospital, Sfax, Tunisia
various sites. The sternum was involved in the majority of patients (6 cases: 20%) (figure). Lesions of keloids were also localized on upper limb (5 cases: 16%), lower limb (5 cases: 16%), face (4 cases: 13%), neck (4 cases: 13%), trunk (2 cases: 6%), shoulder (2 cases: 6%), ear (one case: 3%) and scrotum (one case: 3%).
Twenty cases of the patients were treated with a triple therapy. This therapy includes a silicone gel, a topical corticosteroid and phenolization. Phenolization was performed using a 40% phenol solution with a session per week with an average of 12 sessions (range, 3-60). Four patients were lost to follow-up. A regression more than 50% of the initial keloid scar with satisfaction of the patients or their parents was noted in 10 cases (62%). No systemic manifestation related to the application of phenol was observed.
In five children Triamcinolone acetonide was administered with an intralesional injection 10 mg/cm2 of scar tissue. One session per month with an average of 4 sessions were needed with a favorable outcome in 2 cases.
The other patients (5 cases), were treated only by silicone gel or topical corticosteroids with a favorable evolution in 1 case (20%).
Discussion: Similar to previous reports, our study showed that keloid disorder is more common in older children above age of 10. A female predominance was noted in our study, compared to other series where the sex ratio is almost equal. This finding would be attributable to the more pronounced feminine aesthetic requirement. In some studies, auricular keloids are the most common anatomic locations of keloid lesions in this age group. In our series, ear keloid was seen only in one case. . In our series, etiologies were dominated by burns.
Keloid lesions are difficult to treat with high recurrence rates ranging from 80 to 100%. There have been a limited number of publications in the literature that address treatment of keloids in the pediatric population. Our study confirms the effectiveness and safety of phenolization in combination with silicone gel and topical corticosteroids. Much more research is required in treating childhood keloids.
Conclusion: Our case series is remarkable for the frequency of keloid scars in female children specially after burns and injuries. The pediatric population do not respond to conventional therapy. The successful outcome of phenolization in combination with silicone gel and topical corticosteroids suggests that this combination may be useful in future cases.
Contact Information
Emna Bahloul, MD
Department of Dermatology Hedi
Chaker Hospital 3029 Sfax Tunisia
E-mail: emnabahloul86gmail.com
Telephone number: +21692020209
Fax number: 00216 74 24 26 27- 00216 74 24 45 11


CLINICAL CASE PRESENTATION: Ear Keloid in a Patient with Albinism
Jonel May Breytenbach, MD
ABSTRACT
Background: Keloids are collagenous lesions that extend beyond the original area of injury and is a result of abnormal wound healing. Keloids are limited to humans and although the exact prevalence is unknown, it is reported in 5 – 16% of wounds. Certain races are more susceptible to keloid formation, especially individuals with darker pigmentation, African and Asians. According to literature Caucasians is least effected and no case reports of Albino patients with keloids have been documented
Methods: A 26-year old patient with albinism presented with a 6-month duration of a firm, hard, non-fluctuant, nodular mass on his right ear after being stabbed with a bottle. The lesion extended beyond the borders of the injury. The lesion was excised under local anaesthetic and the specimen was sent to the pathologist for evaluation and the patient received radiotherapy. The histological specimen was then compared to a black patient that developed a firm, hard, non-fluctuant, nodular mass on the left ear of 6 month duration after ear-piercing. Both histological specimens diagnosis consisted of keloids.
Results: The histological specimens were compared after evaluation under magnification and the following results was obtained:
Discussion: There is no significant difference between the two specimens. The patient with albinism’s keloid showed a more advanced and mature lesion compared to the other patient. This could be attributed to the mechanism of trauma being more severe, evoking a more pronounced inflammatory response.
Conclusion: Keloids do exist in Albino patients. The histological report concluded that the collagen fibres were thicker and the epidermis atrophic, suggesting a more aggressive growth in the patient with Albinism. Therefore the histological features shows more advanced and mature features, the pathologist did not attribute the finding as to be significant. It is only a single comparison and needs more investigation.

CLINICAL CASE PRESENTATION: Spontaneous Keloid Syndrome
Abdulhadi Jfri1, Ali Alajmi2ABSTRACT
Background: Keloid scars are benign fibroproliferative tumors that extend beyond the original wound. Spontaneous keloids are those that result without a significant history of trauma. There are many reported cases in the literature.
Objective: This article provides a summary and review of the cases that have been reported with spontaneous keloids and organize them according to the association.
Methods: A literature review was conducted using PubMed and MIDLINE that include English publications cases and case series from May 1955–February 2018.
Results: Spontaneous keloids have been reported mainly in association with certain syndromes such as Rubinstein-Taybi syndrome, Dubowitz syndrome,
- Abdulhadi Jfri, MD. Corresponding author; Dermatology department, McGill University, Montreal, Canada. e-mail: Abdulhadi.jfri@mail.mcgill.ca Address: 1414 Rue Chomedey, H3H 0A2, Montreal, Canada Phone: 438-728-6711
- Ali Alajmi, MD. Dermatology department, McGill University, Montreal, Canada. Email: Ali.Alajmi@mail.mcgill.ca
Noonan’s syndrome, Goeminne syndrome, Bethlem myopathy, conjunctiva-corneal dystrophy, X-linked recessive polyfibromatosis and a novel X-linked syndrome with filamin A mutation. Furthermore, spontaneous keloids were reported in atopic patients and some patients who are medically free.
Conclusion: Spontaneous keloid is diagnosed mainly from history-taking, and it is challenging to confirm whether it is trigged by minimal injury especially if it is single or due to sensibility from underlying medical conditions or genetic disorders.
INTRODUCTION
Keloid scars are benign fibroproliferative tumors that occur in response to injury to the skin of susceptible individuals. Keloid tissue extends beyond the margins of the wound, distinguishing it from hypertrophic scars. [1] Keloids tend to grow symptomless, but still cause pain or itching. They have a functional, aesthetic, or psychosocial impact on patients, as highlighted by quality-of-life studies. [2] Individuals of African,
Hispanic, or Asian descent appear at increased risk for keloid development. [3] Spontaneous keloids, that is those lesions that develop without previous trauma or surgery, are rare. It is generally believed that they are triggered by microtrauma in genetically-susceptible patients. Their etiology is unknown, although they have been described in multiple conditions discussed below.
GENETIC ASSOCIATION
Spontaneous keloids have been associated with certain genetic abnormalities in multiple reports in the literature (Table I). Patients suffering from Bethlem myopathy report multiple keloids on the sternal, upper back, arms, and shoulders. Some of these keloids are spontaneous or occur at a surgical site. These patients are aged 32 to 50 and are both male and female.[4] [5] Individuals who suffer from Rubinstein-Taybi syndrome also report spontaneous keloids [6][7] Extensive analysis 574 patients with Rubinstein-Tabyi syndrome of both male and female patients, aged 3 to 27, revealed multiple cases of keloid eruptions
occurring on the shoulders, back, upper trunk, and arms. [8] Individuals who suffer from Dubowitz syndrome, atopic dermatitis, and Noonan’s syndrome also report sudden onsets of keloids in areas not previously seen. These areas include the right temple and right foot. These patients were young children. [9][10] Goeminne syndrome was named in 1967 after reporter Luc Goeminne, who reported a novel x-linked syndrome in three males of the same family. A 33- year-old male, who exhibited characteristics of congenital muscular torticollis and cryptorchidism, was found to have extensive spontaneous multiple keloids on the chest, back, and arms. [11] In Norway, a mother and her two sons who suffer from conjunctiva-corneal dystrophy report keloidal lesions on their hands and fingers, however the mother did have a history of minimal trauma. [12] A 40-yearold Australian male suffering with X-linked recessive polyfibromatosis and with a personal and family history of keloids, developed a sudden eruption on his trunk and extremities by age 20.[13] A male with novel X-linked syndrome with a mutation in Filamin A (FLNA) that includes cardiac valvular disease with reduction in joint mobility also experienced both a spontaneous keloid eruption at age 6 and one over a surgical incision later on. [14] We reported a case of a female with mental retardation along with orbital hypertelorism, broad nasal bridge, high arched palate and repaired cleft lip also experienced widespread sudden extensive keloids. [15]

MEDICAL CONDITIONS
Spontaneous keloids have been reported in atopic patients. A 24-year-old white female known to have asthma, pollen allergy, and contact allergy to nickel developed 4 eruptive keloidal lesions on the back with a history of trauma. [16] Additionally, a 34-year-old woman with severe asthma and chronic idiopathic angioedema developed two keloidal lesions on the back at age 24 that grew in size over time without trauma. [17]
SPONTANEOUS WITHOUT MEDICAL CONDITIONS
Medically free individuals also report spontaneous keloids. These patients include an 81-year-old male with a single right post-auricular keloid, a 39-yearold male with a sudden eruption on his chest, and a female age 21 with spontaneous keloids on her chest and back. [18][19][20] A 63-year-old man developed eruptive keloids that increased in size and number post-renal transplant but stabilized after a year. A punch biopsy result indicated keloids, which also stained positive for CD68 and factor XIIIa. Though both correlated to lesions of NSF, the patient did not present with classic clinical NSF, had no exposure to gadolinium, and CD34 staining was negative. [21] An analysis of 259 Syrian patients showed keloids in 13.4%, with a significant statistical association between blood group A and spontaneous keloids. [22] Another analysis of 88 Iraqi patients showed that 34% experienced spontaneous eruptions of mostly single keloid. [23] In another report, single spontaneous keloids were seen over the nipple of a 56-year-old male and a 79-year-old female. [24] A report of two sisters, aged 21 and 19, who developed insidious numerous keloids mostly on the chest, back and arms also exists. [25]
MEDICATION
The aromatase inhibitor, Letrezol, is the only medication that can be associated with spontaneous keloid eruption. A patient with a history of keloids experienced new lesions and aggravation of existing ones just two months after starting Letrezol. The keloids eruption stopped when the drug was discontinued. [26]
CONCLUSION
Whereas spontaneous keloids are rare and a challenge to diagnose, the question of genetic links between them and certain mutations is raised due to the many reported cases associated with certain syndromes. However, since there are also reports of eruptions in atopic persons and after Letrezol therapy, as well as incidents without any medical conditions, it is questionable whether there has been some minor trauma of which the patient is unaware.
Keywords: Spontaneous keloids, Spontaneous keloid
Conflicts of interest: The authors have no conflicts of interest that are relevant to the content of this review.
REFERENCES
- Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ. 2003;326(7380):88-92.
- Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297(10):433-8.
- Oluwasanmi JO. Keloids in the African. Clin Plast Surg. 1974;1(1):179-95.
- Echeverria C, Diaz A, Suarez B, Bevilacqua JA, Bonnemann C, Bertini E, et al. Keloids, Spontaneous or After Minor Skin Injury: Importance of Not Missing Bethlem Myopathy. Acta Derm Venereol. 2017;97(2):297-8.
- Collins J, Foley AR, Straub V, Bonnemann CG. Spontaneous keloid formation in patients with Bethlem myopathy. Neurology. 2012;79(21):2158.
- Kurwa AR. Rubinstein-Taybi syndrome and spontaneous keloids. Clin Exp Dermatol. 1979;4(2):251-4.
- Shilpashree P, Jaiswal AK, Kharge PM. Keloids: an unwanted spontaneity in rubinstein-taybi syndrome. Indian J Dermatol. 2015;60(2):214.
- Siraganian PA, Rubinstein JH, Miller RW. Keloids and neoplasms in the Rubinstein-Taybi syndrome. Med Pediatr Oncol. 1989;17(6):485-91.
- Paradisi M, Angelo C, Conti G, Mostaccioli S, Cianchini G, Atzori F, et al. Dubowitz syndrome with keloidal lesions. Clin Exp Dermatol. 1994;19(5):425-7.
- Gulec AT, Karaduman A, Seckin D. Noonan syndrome: a case with recurrent keloid formation. Cutis. 2001;67(4):315-6.
- Goeminne L. A new probably X-linked inherited syndrome: congenital muscular torticollis, multiple keloids cryptorchidism and renal dysplasia. Acta Genet Med Gemellol (Roma). 1968;17(3):439-67.
- Haugen OH, Bertelsen T. A new hereditary conjunctivo-corneal dystrophy associated with dermal keloid formation. Report of a family. Acta Ophthalmol Scand. 1998;76(4):461-5.
- Ly L, Winship I. X-linked recessive polyfibromatosis manifesting with spontaneous keloid scars and Dupuytren’s contracture. Australas J Dermatol. 2012;53(2):148-50.
- Atwal PS, Blease S, Braxton A, Graves J, He W, Person R, et al. Novel X-linked syndrome of cardiac valvulopathy, keloid scarring, and reduced joint mobility due to filamin A substitution G1576R. Am J Med Genet A. 2016;170A(4):891-5.
- Jfri A, Rajeh N, Karkashan E. A Case of Multiple Spontaneous Keloid Scars. Case Rep Dermatol. 2015;7(2):156-60.
- Oittinen HA, O’Shaughnessy M. Multiple nonsyndromic spontaneous keloids in allergic disease. Plast Reconstr Surg. 2007;119(2):762-3.
- 17. McCabe J, Blades Z, McGrath EE. A spontaneous skin lesion. CMAJ. 2008;179(12):1297-9.
- Monarca C, Maruccia M, Palumbo F, Parisi P, Scuderi N. A rare case of postauricular spontaneous keloid in an elderly patient. In Vivo. 2012;26(1):173-5.
- Alonso PE, Rioja LF. Spontaneous resolution of a keloid. Plast Reconstr Surg. 2011;128(2):98e-9e.
- Kerfant N, Gasnier P, Boloorchi A, Uguen A, Hu W. [Spontaneous keloids: about a rare case]. Ann Chir Plast Esthet. 2011;56(4):339-41.
- Bremmer M, Deng A, Martin DB. Spontaneous eruptive keloid-like cutaneous lesions in a renal transplant patient: a form of nephrogenic systemic fibrosis? J Dermatolog Treat. 2009;20(1):63-6.
- Shaheen A, Khaddam J, Kesh F. Risk factors of keloids in Syrians. BMC Dermatol. 2016;16(1):13.
- Sharquie KE, Al-Dhalimi MA. Keloid in Iraqi patients: a clinicohistopathologic study. Dermatol Surg. 2003;29(8):847-51.
- Finlay GH, Stoughton RB. Spontaneous keloid with unusual histologic features. AMA Arch Derm. 1955;71(5):599-601.
- Mandal A, Imran D, Rao GS. Spontaneous keloids in siblings. Ir Med J. 2004;97(8):250-1.
- Meade C, Smith S, Makhzoumi Z. Eruptive keloids associated with aromatase inhibitor therapy. JAAD Case Rep. 2015;1(3):112-3.
Basic Science of Keloid Disorder
Gene Expression in Keloids and Pleiotropic Effects of Risk Factors for Fibroproliferative Disease
Shirley Brody Russell, PhD
ABSTRACT
Elucidating fibrosis requires useful models for study. One widely used model is cell culture, which permits detailed examination of cell behavior and effects of specific mediators on gene expression. While it lacks in vivo context, culture manipulations can include hormones and growth factors from fibroblast and nonfibroblast cell types to simulate the in vivo environment. In the early 1970’s when we began to study cultured fibroblasts from keloids, few studies were being done. Our initial findings showed no differences in growth or gene expression between cells derived from keloids versus normal skin and scar. Because the cells were being fed daily with medium containing 10% fetal bovine serum, we thought that the culture medium was simulating a wound healing environment and to see differences we might need to add modulators that played a role in wound termination. When we added physiological concentrations of hydrocortisone to the culture medium we found that keloid fibroblasts were resistant to down regulation of collagen and elastin synthesis whereas normal fibroblasts were not.[1-3]. We also learned that only fibroblasts from the keloid nodule were resistant, and resistance was maintained throughout the culture lifetime suggesting an epigenetically altered program of gene expression. The mechanism of this resistance to hydrocortisone has not been determined. Although we were unable to definitively show it, the altered glucocorticoid response may be due to differential expression and/or activation of one or more members of the AP-1 family of transcription factors [3-5]. Targeting c-Jun, a prominent member of the family, which has recently been suggested to be a central mediator of fibrosis [6], may help to develop new and effective therapies.
Over the past 20 years we and others have used microarrays to expand studies of gene expression in keloid fibroblasts to genome wide expression profiling. Differences in expression of many genes that may play a role in fibrosis have been observed between keloid and normal fibroblasts [7-10]. Although the genes identified by different laboratories have varied, certain common patterns have emerged, some of which have also been observed in tissue. There is evidence for increased Wnt and Notch signaling, decreased activity of several matrix metalloproteinases, altered expression and activity of genes in the TGFb family and increased collagen and fibronectin expression under a variety of cell culture conditions. Many different pathways have been implicated but the overall network of misregulation has not been defined. We are currently testing the effects of silencing and overexpressing candidate keloid genes on the altered pattern of expression in keloid fibroblasts.
While most gene expression profiling has been done with coding genes, an increasing number of studies are examining the contribution of noncoding RNAs including microRNAs and long noncoding RNAs are being examined [11]. These studies point toward regulation of multiple coding genes by a single RNA.
An epigenetically altered wound healing program is supported by culture stability, limitation of the altered program to the keloid nodule, differential methylation and histone modification of some differentially expressed genes and reversal of part of the fibrotic phenotype by inhibitors of methylation and histone deacetylation [12]. Important contributions to the study of an epigenetically altered state have been provided by Jones, et al. who has defined a pattern of differential methylation in fibroblasts from keloid tissue[13].
Although the fibroblast plays a central role in keloids, other cell types that regulate fibroblast gene expression, such as keratinocytes, leukocytes, melanocytes, and mast cells may play critical roles in keloid pathogenesis [14]. Gene expression profiling in cultured keloid keratinocytes reveals a pattern similar to that of the activated keratinocytes in healing wounds, that is regulated by TGFb1 and may promote fibrosis [10, 15]. Another cell type of interest Type 2 helper T cell that secretes profibrotic IL4 and IL13 [16].
Studies of gene expression in specific cell types and in keloid tissue will continue to provide important information about the roles of genes and pathways involved in keloid pathogenesis and the opportunity to test effects of specific genes on the keloid phenotype. Recent efforts to create engineered tissue equivalents and animal models to examine gene expression are helping to elucidate the overall program of wound healing and keloid pathogenesis.
REFERENCES
- Russell SB, Trupin JS, Myers JC, Broquist AH, Smith JC, Myles ME, et al. Differential glucocorticoid regulation of collagen mRNAs in human dermal fibroblasts. Keloid-derived and fetal fibroblasts are refractory to down-regulation. J Biol Chem. 1989;264(23):13730-5. PubMed PMID: 2760040.
- Russell SB, Trupin JS, Kennedy RZ, Russell JD, Davidson JM. Glucocorticoid regulation of elastin synthesis in human fibroblasts: down-regulation in fibroblasts from normal dermis but not from keloids. J Invest Dermatol. 1995;104(2):241-5. PubMed PMID: 7829880.
- Xu J, Russell JD, Trupin JS, Russell SB, editors. Transcriptional Regulation of collagen genes by glucocorticoids: normal and keloid fibroblasts differ in nuclear proteins that bind the GRE, CRE, and TRE. Fourth International Conference on the Molecular Biology and Pathology of Matrix; 1992; Philadelphia, PA.
- Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990;249(4974):1266-72. PubMed PMID: 2119054.
- Kim CW, Suh SI, Sung SH, Lee IK, Lee KS. A transcriptional factor decoy against AP-1 suppresses TGF-beta1- induced type I collagen gene expression in cultured keloid fibroblasts. Journal of dermatological science. 2005;37(1):49-51. PubMed PMID: 15619434.
- Wernig G, Chen SY, Cui L, Van Neste C, Tsai JM, Kambham N, et al. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA. 2017; 114(18):4757-62. Epub 2017/04/21. doi: 10.1073/ pnas.1621375114. PubMed PMID: 28424250; PubMed Central PMCID: PMCPMC5422830.
- Smith JC, Boone BE, Opalenik SR, Williams SM, Russell SB. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol. 2008;128(5):1298-310. PubMed PMID: 17989729.
- Seifert O, Bayat A, Geffers R, Dienus K, Buer J, Lofgren S, et al. Identification of unique gene expression patterns within different lesional sites of keloids. Wound Repair Regen. 2008;16(2):254-65. PubMed PMID: 18282266.
- Jumper N, Hodgkinson T, Paus R, Bayat A. Site-specific gene expression profiling as a novel strategy for unravelling keloid disease pathobiology. PLoS One. 2017;12(3):e0172955. Epub 2017/03/04. doi: 10.1371/ journal.pone.0172955. PubMed PMID: 28257480; PubMed Central PMCID: PMCPMC5336271.
- Hahn JM, Glaser K, McFarland KL, Aronow BJ, Boyce ST, Supp DM. Keloid-derived keratinocytes exhibit an abnormal gene expression profile consistent with a distinct causal role in keloid pathology. Wound Repair Regen. 2013;21(4):530-44. Epub 2013/07/03. doi: 10.1111/wrr.12060. PubMed PMID: 23815228.
- Herter EK, Xu Landen N. Non-Coding RNAs: New Players in Skin Wound Healing. Advances in wound care. 2017;6(3):93-107. Epub 2017/03/16. doi: 10.1089/ wound.2016.0711. PubMed PMID: 28289554; PubMed Central PMCID: PMCPMC5346954.
- Russell SB, Russell JD, Trupin KM, Gayden AE, Opalenik SR, Nanney LB, et al. Epigenetically altered wound healing in keloid fibroblasts. J Invest Dermatol. 2010;130(10):2489-96. PubMed PMID: 20555348.
- Jones LR, Young W, Divine G, Datta I, Chen KM, Ozog D, et al. Genome-Wide Scan for Methylation Profiles in Keloids. Disease markers. 2015;2015:943176. Epub 2015/06/16. doi: 10.1155/2015/943176. PubMed PMID: 26074660; PubMed Central PMCID: PMCPMC4446486.
- Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: The paradigm of skin fibrosis – Pathomechanisms and treatment. Matrix biology : journal of the International Society for Matrix Biology. 2016;51:37-46. Epub 2016/02/05. doi: 10.1016/j. matbio.2016.01.013. PubMed PMID: 26844756; PubMed Central PMCID: PMCPMC4842154.
- Hahn JM, McFarland KL, Combs KA, Supp DM. Partial epithelial-mesenchymal transition in keloid scars: regulation of keloid keratinocyte gene expression by transforming growth factor-beta1. Burns & trauma. 2016;4(1):30. Epub 2016/08/31. doi: 10.1186/ s41038-016-0055-7. PubMed PMID: 27574697; PubMed Central PMCID: PMCPMC4994224.
- Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7(5):e1002003. Epub 2011/05/19. doi: 10.1371/journal.ppat.1002003. PubMed PMID: 21589896; PubMed Central PMCID: PMC3093361.
Genetics of Keloid Disorder
Ernst J. Reichenberger PhD
ABSTRACT
It has long been suspected that there is a strong genetic basis contributing to the development of keloid scars. Keloids affect predominantly individuals of African and Asian descent and are found to a lesser degree in white Europeans. Prevalence in some African populations may be up to 6%. More than 30% of Nigerian keloid patients participating in our study report other family members with keloids.
However, the complexity of processes during wound healing, the age-related onset of keloids and incomplete penetrance in keloid families as well as the unknown mode of inheritance have been obstacles in identifying the genetic basis of keloids in the past. Because of the large number of orchestrated events during wound healing we expect a large number of gene variants that partake in keloid formation.
Familial keloids are most likely passed on in a dominant additive or complex trait. We believe that more than one disease-causing variant is needed in a predisposed individual to develop keloids with one variant being the major effector for phenotype development. This may explain the high rate of carriers that can be found in keloid families. In addition,
somatic mutations in keloid tissue may contribute to the spectrum of keloid severity within a family.
Wound healing and scar formation in keloids is accompanied by continuous inflammation on the scar borders, by upregulated extracellular matrix deposition of fibroblasts and by reduced capability to resorb scar tissue. Therefore, studies that focused on any of these events identified abnormal gene expression of numerous targets, however, underlying causative effects that lead to these events have been elusive.
With recent advances of genetic methods it is increasingly possible to identify such targets. We have, for example, used a combination of linkage analysis and whole exome sequencing to identify a number of candidate variants that are passed on in individual families. We identified a non-synonymous variant in ASAH1 (N-acylsphingosine amidohydrolase) as a rare candidate. ASAH1 is expressed mostly in the epidermis and is one of the enzymes that control the ratio of ceramide and sphingosine. ASAH1 is involved in tumor formation and is a potential molecular target to control cancer progression. While we expect to identify more variants that affect the coding regions of wound healing genes, epigenetic studies will be needed to investigate regulatory regions that control the temporal and spacial gene expression profile of cells involved in wound healing. Environmental factors, which include the overall health and immune status of a keloid patient, may also determine whether a wound becomes keloidal or heals normally. Lastly, factors that contribute to the age-related onset of keloids are still elusive.
In summary, methods are now available to study the genetic basis of keloids and first candidate genes and regulatory regions have been identified. Functional studies are needed to determine the effect of those genetic variants on individual cellular processes and on wound healing overall. The goal should be to develop personalized treatment for patients as we know that not all patients respond to treatment and the recurrence rate is still high.
Progress in Keloid Animal Models
Dorothy M. Supp, PhD
Shriners Hospitals for Children-Cincinnati,
Research Department, and The University of
Cincinnati College of Medicine, Department of
Surgery, Cincinnati, Ohio, USA
Keloid scarring is disfiguring fibroproliferative disorder that can significantly impair quality of life in affected individuals. Keloids result from an abnormal wound healing response that involves excessive and prolonged deposition of extracellular matrix (ECM), particularly collagen. In spite of decades of research that has advanced our understanding of wound healing, the mechanisms that initiate keloid scarring remain poorly understood. Although there are multiple therapies currently available, keloids remain one of the most challenging skin conditions to treat. Ideally, effective therapies would be based on a detailed understanding of the underlying pathology, and would be developed and tested using preclinical animal models. However, keloid scarring is unique to humans, which has significantly hindered research efforts aimed at prevention and therapeutic intervention. It is not clear why humans form keloid scars while animals do not. Keloid etiology is complex, and there are numerous physiological differences between humans and rodents, the most commonly used research animals, as well as differences in pathology and responses to injury. In spite of these differences, mice are commonly used in wound healing studies, and they can form thickened scars under certain experimental circumstances. Pigs are increasingly used in wound healing studies, as pig skin more closely resembles human skin in thickness and hair density, and some pig breeds develop raised scars resembling hypertrophic scars (HTS) under certain wounding conditions. However, features observed in human keloids, such as continued growth beyond the wound margin, have not been reported in any porcine wound model. The only animal known to naturally develop extreme fibroproliferative scarring is the horse, which can develop a type of scarring known as exuberant granulation tissue (EGT). Similar to human keloids, EGT in horses is raised and extends beyond the wound bed, and fibroblasts of EGT display overproduction of ECM. However, in contrast to keloids, EGT frequently occurs prior to completion of wound re-epithelialization and keloidal collagen is not observed. Recent studies have utilized tissue engineering approaches that enable grafting of scaffolds containing keloid-derived fibroblasts and/ or keratinocytes to mice, resulting in mouse-human hybrid animal models. Although some of these models display similarities to human keloids, such as deposition of keloidal collagen and aberrant gene expression, they are limited in that immunodeficient mice are required to enable engraftment of human cells. Not surprisingly, there is mounting evidence that the immune system plays an active role in formation of abnormal scars, including keloids. Future animal models may take advantage of humanized mice with immune systems reconstituted using keloid patient-derived immune cells. Such models, when combined with grafted tissues prepared using keloid scar-derived cells, might enable investigation of the complex interactions of systemic and local factors that combine to promote keloid scar formation. Although such models may be impractical for large-scale drug screening, they may reveal critical mechanisms of keloid pathology that can be leveraged for development of targeted therapies.
From Experimental Data to a Numerical Model of “Keloid-Surrounding Skin” Composite Structure
Sutula D.;2, Sensale M. 3, Chambert J.1;2, Chouly F1;4,
Lejeune A.1;2, Rolin G.5;6, Bordas S.7, Jacquet E.1;2
Keloid scarring unfortunately results in an unsightly and uncomfortable fibrotic tissue. This tumor-like structure is elevated, firm and rigid unlike healthy skin which is elastic and deformable. Furthermore, it is now well documented that they appear on pro-keloid body sites, such as chest or shoulders [1] subjected to strong mechanical deformation due to body motion [2]. When they trigger keloids, mechanical forces are the dark side of a parameter yet present in the physiological state of skin. But interestingly, mechanical forces can also be turned into positivity when they are used to prevent keloids development or recurrence [3]. That can be done by the use of medical devices usually based on compression. However, designs of such medical devices are basic and do not to take fully into consideration the complex mechanical status of keloids. Thus, the stress fields which initially triggered and maintain the fibrosis are not fully elucidated. Precise knowledges about the mechanical characterization of the “keloid – surrounding skin” composite (KSSC) structure are needed and could strongly lead to medical devices improvement.
- Univ. Bourgogne Franche-Comté;
- FEMTO-ST Institute, UFC/CNRS/ENSMM/UTBM, Department of Applied Mechanics, Besançon, France;
- Polytechnic University of Turin, Italy
- Laboratoire de Mathématiques de Besançon, France;
- INSERM CIC-1431, University Hospital of Besançon, Clinical Investigation Center, Fédération Hospitalo-Universitaire INCREASE, LabEx LipSTIC, F-25000 Besançon, France;
- Univ. Bourgogne Franche-Comté, INSERM, EFS BFC, UMR1098, Interactions Hôte-Greffon-Tumeur/Ingénierie Cellulaire et Génique, F-25000 Besançon, France;
- University of Luxembourg, Institute of Computational Engineering, Luxembourg.
The objective of this study was to model both numerically and experimentally (the KSSC structure in order to 1- qualify stress fields related to mechanical solicitation of tissues and 2- validate the modelization method with both experimental and numerical tests through a sensitivity analysis.
First, a silicon molding was made from an upper-left arm keloid. The molding was then observed by 3D-microscopy (Alicona Infinite Focus: optical form and roughness measuring system). A 3D numerical model has been obtained by CAD software (Rhinoceros®-3D ver. 3.0) from the filtered images and then analyzed by a finite element method (ANSYS® Sortaware) with hyper-elastic materials behaviors. Stress fields calculated in silico were secondly compared to in vitro experimental data. Secondly, keloid mechanical properties and surrounding skin were measured by a uniaxial test with a home-made innovative device [4]. This assay can be approximated as a tensile test of the surface of the skin and tissue deformation was assessed by digital image correlation method. Indeed, a random speckled pattern of painting was drawn on skin surface before the test and spot movements were recorded by a digital camera positioned perpendicular to the measurement area. Displacement fields were determined from the analysis of speckled pattern motion. From these biomechanical assays, a numerical model of KSSC structure has been built. Five parameters of the Mooney-Rivlin hyperelastic model has been identified from the experimental stress-strain curves for keloid and healthy skin, and all the components of the stress fields resulting from the traction test has been obtained. The numerical results showed a relative high error between the predicted force calculated from simulation of the mechanical test and the resulting force measured on the force sensor.
An original investigation method has begun to be developed here from both numerical analysis and in vivo experiments. This investigation deals with the mechanical modelization of a keloid scar surrounded by normal tissue: in vivo mechanical measurements and image acquisition led to the quantification of keloid stress strain and motion fields. These data allowed the construction of a KSSC numerical model. This model was analyzed by finite element method in order to link strain to deformation in the structure. In this way, the numerical model was used to obtain predictive values of both strain and motion.
For instance, it remains some differences between theoretical data and those from in vivo assays used as control. Supplementary input data are needed, especially concerning the mechanical comportment of the keloid border as a well as the contribution of sub-cutaneous attachment in keloid biomechanic. In order to improve the numerical simulation, an intermediate physical model of the KSSC is under development. The aim is to build a KSSC made of a fully characterized artificial material intended to be used as a physical keloid phantom. This phantom will be used to test biomechanical hypotheses and will contribute to understand in vivo KSSC and further validate the KSSC numerical model. Beyond the quantification of stress fields, the next step will also consist in elucidating their nature (tension, shear stress…). The full understanding of the mechanical environment surrounding keloid will help to design efficient device able to contain or prevent this tumor-like growth.
Funding source
This work was funding by the “Université de Franche-Comté” and “Région Bourgogne Franche-Comté” (Grant 2017Y-06397).
Contact information:
Dr. Gwenaël Rolin, PhD
Hospital Research Engineer
INSERM CIC-1431, University Hospital of
Besançon, Clinical Investigation Center
2 place St Jacques – 25000 Besançon
grolin@chu-besancon.fr
Office: +33 (0)3 81 21 91 64
Mobile: +33(0)6 84 25 77 92
REFERENCES
- Ogawa, R., & Orgill, D. (2009). Mechanobiology of Cutaneous Wound Healing and Scarring, in Bioengineering Research of Chronic Wounds. Berlin Heidelberg: Springer.
- Ogawa, R., Okai, K., Tokumura, F., Mori, K., Ohmori, Y., Huang, C., Hyakusoku H and Akaishi, S. The relationship between stretching/contraction and pathologic scarring : the important role of mechanical forces in keloid generation (2012) Wound Repair and Regeneration. 20:149-157.
- Park TH and Rah DK. Successful eradication of helical rim keloids with surgical excision followed by pressure therapy using a combination of magnets and silicone gel sheeting. Int Wound J (2017) 14:302-306.
- Jacquet, E., Joly, S., Chambert, J., Rekik, K., & Sandoz, P. Ultra-light extensometer for the assessment of the mechanical properties of the human skin in-vivo (2017) Skin Research and Technology. 4:531-538.
Keloids: Proliferative or Inflammatory Disorder?
Dr. Nangole Wanjala F
Senior lecturer, Dept of surgery,
University of Nairobi
ABSTRACT
Introduction: Keloids have always been defined as dermatoproliferative disorder of the skin. The disorder has been characterized with excessive deposition of the collagen fibers by the fibroblasts Histology has also shown the keloids specimens to have high concentrations of fibroblasts and myofibroblasts as compared to the normal skin. Recent studies have however shown keloid specimens to have a high concentration of the inflammatory cells including lymphocytes, macrophages and mast cells. Patients with keloids have also been shown to have high concentration of inflammatory cytokines than the normal patients in the serum. Patients with inflammatory conditions such as liver cirrhosis in have also been noted to have high incidences of keloids. More importantly normal fibroblasts in vitro studies with inflammatory cells such as mast cells have been shown to produce large amounts of collagen fibers similar to what is found in patients with keloids.
Clinically some patients have shown keloids symptoms such as pains and itchiness worsened by eating particular foods. In this presentation we present the evidence that suggests keloids to be as a result of abnormal inflammatory response that stimulate fibroblasts to produce abundance collagen as opposed to being primarily a fibroblast proliferative disorder.
Advancing the Study of Keloids with In-Vitro Modeling and Single-Cell Sequencing
Scott MacDonnell1, Ted Kaplan1, Michael Bachelor2, Jennifer Molignano2, Qin Ruan1, Rebecca Peyser1, Gabor Halasz1, Ernst Reichenberger3, Lori Morton1
Background: Keloids are a form of abnormal wound healing and manifest as benign, dermal fibroproliferative tumors – “a confused scar that does not know when to stop growing.” While keloid scars are not malignant, the morbidity imparted by keloid scars cannot be overlooked. Keloids can become both itchy and painful, with significant physiological (joint mobility, obstruction of sensory organs) and psychological impact on quality of life. A major challenge in understanding keloid biology is the lack of translatability between mice and humans in addition to challenges with 2D culture systems that lack the necessary architectural and multi-cellular aspects of human skin. The purpose of the current study was to generate a full thickness skin model of human keloid scars and in parallel pilot methods to isolate and store skin cells for single cell sequencing studies.
- Regeneron Pharmaceuticals Inc, Department of Cardiovascular/Renal/Fibrosis Research, Tarrytown, NY
- MatTek Corporation, Ashland, MA
- University of Connecticut Health, Farmington, CT
Methods: Normal human dermal fibroblasts (NHDF) were cultured in a collagen gel onto which normal human epidermal keratinocytes were then seeded. Constructs were raised to the air-liquid interface and cultured for up to 14 days in serum-free culture medium to produce stratified, differentiated fullthickness skin equivalents, referred to as EpiDermFT™ (EFT-400, MatTek Corporation). EpiDermFT tissues were constructed using keloid-derived or healthy fibroblasts under healthy neonatal or adult keratinocytes. Prepared in this fashion, keloid EpiDermFT tissues were compared with TGF-β- stimulated and unstimulated healthy EpiDermFT tissues using histologic, immunohistochemical, and NGS endpoints. In a separate experiment, mouse skin single-cell preparations were submitted for bulk or 10X NGS after various storage conditions and analyzed for relative stability of gene expression patterns vs. freshly isolated cells.
Results: EpiDermFT produced with keloid-derived fibroblasts display gross morphologic abnormalities, unique immunohistochemical staining patterns for key (myo)fibroblast-associated markers, and a pro-fibrotic gene signature (e.g. upregulated Col I, α-SMA, Activin A, Fibronectin); these observations were not fully-recapitulated in TGF-β-stimulated healthy samples. Mouse skin samples were shown to maintain their gene expression profiles when frozen or stored as tissue or cell suspension in a storage buffer for 1-2 days, displaying minimal changes from baseline observations in freshly-isolated cells.
Conclusions: Development of an in-vitro 3D keloid organ culture system may allow for the evaluation of novel therapeutics in addition to modeling the biologic relevance of novel keloid associated variants. Ensuring the quality and stability of stored isolated skin cells may allow for single cell sequencing studies to be conducted on cells collected anywhere in the world. Together, these two approaches provide us with a potential platform for the genetic and molecular study of human keloid/skin biology.
Contact Information:
Scott MacDonnell, PhD.
Senior Staff Scientist
Department of Cardiovascular/Renal/Fibrosis Research
Regeneron Pharmaceuticals
777 Old Saw Mill River Road
Tarrytown, NY 10591
914-255-2347
scott.macdonnell@regeneron.com
Halofuginone vs TGF-Mediated Fibrosis: in Vitro Investigations on Site-Specific Keloid Fibroblasts
Marty P. 1,2,3, Chatelain B.2, Lihoreau T.1, Secomandi E.4, Meyer C.2, Isidoro Ciro4 and Rolin G.1,3
Keloids scars are fibrotic tumors resulting from an abnormal proliferation of cutaneous tissue beyond the original wound margins. Considered as chronic inflammatory diseases, keloids do not regress over time, exhibit high recurrence after surgery and do not currently benefit from effective therapy [1].
On one hand, fibroblasts are one the key cells involved in keloid pathogenesis. Keloid fibroblasts exhibit a fibrotic phenotype compared to their healthy counterpart and strongly differ according their location in the keloid center or periphery [2]. On the other hand, halofuginone (HF) has been described as a promising anti-fibrotic and anti-inflammatory molecule which inhibits Smad3 phosphorylation downstream of the TGF-β signaling pathway and prevents of Th17 cell differentiation [3]. However, the potential of HF on keloids [4] has not been investigated yet despite evidences about HF’ role as TGF reversing agent of the fibrogenesis progression.
The objective of our work was to evaluate for the first time in vitro the anti-fibrotic effect of HF on human site-specific keloid fibroblasts (KFs).
- INSERM CIC-1431, University Hospital of Besançon, Clinical Investigation Center, Fédération Hospitalo-Universitaire INCREASE, LabEx LipSTIC, F-25000 Besançon, France;
- University Hospital of Besançon, Department of Maxillo-Facial surgery, Stomatology and Odontology, F-2500 Besançon, France;
- Univ. Bourgogne Franche-Comté, INSERM, EFS BFC, UMR1098, Interactions Hôte-Greffon-Tumeur/Ingénierie Cellulaire et Génique, F-25000 Besançon, France;
- Laboratory of Molecular Pathology and Nanobioimaging, Department of Health Sciences, Università del Piemonte Orientale, Italy
To this aim, keloid tissue samples were collected during surgery from patients suffering from earlobe keloid [“Scar Wars” Clinical Study – Clinical Trial NCT03312166]. Then, fibroblasts (FK) were extracted from the center (FKc) and periphery (FKp) of resected tissues. FKc and FKp were stimulated by TGF-β and the HF was assessed considering its effect on migration [wound healing assay], proliferation [MTT and Ki- 67 expression], myofibtoblasts differentiation [α-sm expression], ECM synthesis [RT-PCR and ELISA on procollagen type I, MMP-1, TIMP-1] and 3D
remodeling [collagen gel retraction assay].
Although cells behavior (i.e proliferation and response to TGF) have been shown very different for FKc compared to FKp, halofuginone was shown to overcome TGF-β effect on human site-specific keloid fibroblasts in a dose-effect way. HF was also able to reduce both FK over-proliferation and FK migration mediated by TGF-β. HF limited proliferation of TGF-β stimulated Fkc and FKp and decreased the number of proliferative Ki-67+ cells. HF slowed down migration of FKc and FKp as well as regulated the capacity of both cell lines to synthesize and remodel ECM.
To our knowledge, the anti-fibrotic effect of HF on keloid cells was reported here for the very first time. Our results proved that HF is a relevant drug for treatment or prevention of keloid scars. Next works will address the anti-inflammatory effect of HF on keloids both on in vitro and animal complex models in order to move forward from bench to bedside.
Contact information
Dr. Gwenaël Rolin, PhD
Hospital Research Engineer
INSERM CIC-1431, University Hospital of
Besançon, Clinical Investigation Center
2 place St Jacques – 25000 Besançon
grolin@chu-besancon.fr
Office: +33 (0)3 81 21 91 64
Mobile: +33(0)6 84 25 77 92
Funding source: This work was funding by the “University hospital of Besançon” and “Région Bourgogne Franche-Comté” (Grant API3A2016).
REFERENCES
- Lee HJ and Jang YJ. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int J Mol Sci. 2018. 19, 711.
- Jumper N, Hodgkinson T, Paus R and Bayat A. Site-specific gene expression profiling as a novel strategy for unravelling keloid disease pathobiology. PLoS One. 2017. 12:e0172955.
- Luo Y, Xie X, Luo D, Wang Y and Gao Y. The role of halofuginone in fibrosis: more to be explored? J Leukoc Biol. 2017. 102:1333-1345.
- Lista S and Emanuele E. Potential therapeutical effects of topical halofuginone hydrobromide in keloid management. Med Hypotheses. 2007. 69:707.
Role of Surgery in Treatment of Keloid Lesions
Optimization of Surgical Management for the Treatment of Auricular Keloids
Tae Hwan Park., MD, PhD
Department of Plastic and Reconstructive Surgery, CHA Bundang Medical Center, CHA University, Songnam, Republic of Korea
ABSTRACT
Keloids are often resistant to treatment with high recurrence rates. Ears are one of the most commonly involved anatomical areas and auricular keloids usually occurs following ear piercing with an incidence of 2.5 %. Their cosmetic deformity and psychological trauma are very troublesome to the patients.
In my opinion, surgical excision followed by adjuvant therapy is gold standard for auricular keloids in Asian population. In terms of surgical management of keloids, balancing between free surgical margins and minimizing tension is crucial to prevent keloid recurrence. In addition, anatomical locations in ears and planned postoperative adjuvant therapy should be carefully considered to provide better outcomes for the treatment of auricular keloids.
Contact information:
Tae Hwan Park., MD, PhD
Department of Plastic and Reconstructive Surgery
CHA University College of Medicine
59 Yatap-ro, Bundang-gu, Seongnam,
Gyeonggi 13496, Korea
Tel: +1-240-205-2650
E-mail: hard-piano@hanmail.net
Conflict of Interest: None.
Financial Disclosures: None.
Ethical Approval
This study was approved by the institutional review board of the CHA University and conducted in accordance with the Declaration of Helsinki.
Intralesional Surgery in Combination with Contact Cryotherapy
F. B. Niessen, MD,PhD,
F. Heijsters, MD, T. Fakkel, MD.
Department of Plastic, Recosntructive and
Handsurgery, VU University Medical Center,
Amsterdam, Netherlands.
ABSTRACT
Excessive scar formation is a burden for patients and a challenge to treat for the specialist. Especially for keloids a good therapy is lacking and recurrence rates are high. Although excision and HDR brachytherapy seems to be a good option, it still shows recurrences especially in darker skin types, is not everywhere available and is an expensive therapy.
In recent years cryotherapy for keloids has become more popular. With needles like Cryoshape, or more simple as contact cryo therapy, the bulk of the keloids can get reduced.
A drawback is the number of treatments. In the VU University Medical Center in 2017 we started a pilot study to excise the keloids intralesionally, stich subcutaneous and intracutaneous with Monocryl® and end up with contact cryo.
The contact cryo was done in two cycles of 10 seconds freezing and thawing.
Preliminary results regarding recurrence rates and complications will be shown at the congress.
A Dermatologic Surgeon’s Approach to Keloids
Jürg Hafner, MD, Dept. of Dermatology,
University Hospital of Zurich, Switzerland
During my residency I have learnt two strategies to treat keloids:
- Cryotherapy and immediate triamcinolone infiltration into the softened tissue. This treatment was repeated 4x – 12x at a 4-weekly interval.
- Excision (often intralesional excision) and adjuvant radiotherapy with 6x 2 Gy in the first two postoperative weeks.
In my personal experience, regimen (A) remained ineffective in most instances. After an initial improvement that commonly lasted 2 weeks, the keloid usually recurred to the original size. Until the patient came again after 4 weeks, the lesion looked virtually unchanged.
Regimen (B) gave good results, when the excised and irradiated lesion in fact was a hypertrophic scar (and not a keloid). Keloids tended to recur in the first 3-9 months.
Since eight years our team has changed the concept:
- In multiple small keloids we infiltrate the lesions with ad admixture of 40 mg triamcinolone and 150 mg 5-fluorouracil. During the first 4 months the interval is 4 weeks, and thereafter 8 weeks. The majority of patients respond well with long lasting remissions after a total of 8 sessions.
- Large keloids are excised or laser-abladed (ear keloids), with postoperative radiotherapy, followed by triamcinolone-5FU infiltrations. The majority of patients respond well with long lasting remissions
Typical examples of keloid treatment are shown and discussed.
For a better evaluation of the different modalities, randomized controlled studies are indispensable.
Contact information:
Prof. Dr. med. Jürg Hafner, MD
Division in-patient dermatology and surgical dermatology
Department of Dermatology,
University Hospital of Zurich, Switzerland
juerg.hafner@usz.ch
Role of Adjuvant Radiation Therapy in Management of Keloid Lesions
Jonathan Tsao MD
ABSTRACT
Keloids are a benign fibroproliferative disease that is often associated with significant cosmetic impairment and local symptomatology. The risk of recurrence after local excision is high and the literature supports the role of postoperative adjuvant radiotherapy in significantly decreasing this risk. The clinical procedure and results of external x-ray and electron beam radiotherapy, and LDR and HDR brachytherapy will be examined and compared.
Topic Code: External Radiotherapy, Brachytherapy, Keloids
Contact information:
Division of Radiation Oncology,
Carlo Fidani Peel Regional Cancer Centre,
Trillium Health Partners,
2200 Eglinton Avenue West, Mississauga,
ON Canada L5M 2N1
Tel: (905) 813 1100 x4803
Fax: (905) 813 3962
Email: jonathan.tsao@thp.ca
Algorithm for Treatment of Chest Wall Keloids. Report of a Large Study from Peking Union Medical College Hospital
Xiao Long, MD
Division of Plastic Surgery, Peking Union Medical College Hospital
ABSTRACT
Keloids are common in the Asian population. Multiple or huge keloids can appear on the chest wall because of its tendency to develop acne, sebaceous cyst, etc. It is difficult to find an ideal treatment for keloids in this area due to the limit of local soft tissues and higher recurrence rate. This study aims at establishing an individualized protocol that could be easily applied according to the size and number of chest wall keloids.
A total of 445 patients received various methods (4 protocols) of treatment in our department from September 2010 to September 2016 according to the size and number of their chest wall keloids. All of the patients received adjuvant radiotherapy in our hospital. Patient and Observer Scar Assessment Scale (POSAS) was used to assess the treatment effect by both doctors and patients. With mean follow-up time of 13 months (range: 6–18 months), 362 patients participated in the assessment of POSAS with doctors. Both the doctors and the patients themselves used POSAS to evaluate the treatment effect.
The recurrence rate was 0.83%. There was an obvious significant difference (P<0.001) between the before-surgery score and the after-surgery score from both doctors and patients, indicating that both doctors and patients were satisfied with the treatment effect. Our preliminary clinical result indicates that good clinical results could be achieved by choosing the proper method in this algorithm for Chinese patients with chest wall keloids. This algorithm could play a guiding role for surgeons when dealing with chest wall keloid treatment.
Abbreviations: IMA = internal mammary artery,
POSAS = Patient and Observer Scar Assessment Scale.
Keywords: algorithm, chest wall, keloid, surgery
Subcutaneous Surgical Resection of Keloid Lesions
Wang Youbin, MD
Four Decades of Experience with Radiation Therapy in Treating Keloid Lesions at the Royal Marsden Hospital, London, St George’s University Hospital, London and Cancer Centre London. Current Procedures and Potential for Future Developments
Dr John Glees (Clinical Oncologist) MD, FRCR,
DhanaJayan Kothandan (Radiographer) MSc,
Mhairi Furness (Radiographer) MSc, Dr Andrew
Fleming (Plastic Surgeon), MB, ChB, FRCS and Dr
Henry Weatherburn (Physicist), PhD
Cancer Centre London, Parkside,
London SW19 5NB, UK.
INTRODUCTION
The lead author has been involved with the treatment of Keloids and Keloid scars using superficial radiotherapy for more than 40 years at the Royal Marsden Hospital, London, St Georges University Hospital, London and, latterly (with his current colleagues), at Cancer Centre London. A description of procedures employed, results of treatment to large nubers of patients and developments currently underway will be given.
METHOD
(1) Treatment of Excised Keloids
The use of superficial radiotherapy (SRT) to treat Keloids in order to avoid post-surgical recurrence is well established [1].
For those patients wishing to be rid of unsightly keloids as soon as possible, we work closely with our plastic surgeon. The treatment protocol is for each patient presenting with keloid to be photographed, measurements taken, Keloid thickness assessed and arrangements made for the Keloid to be removed: only one postoperative superficial single dose of 10Gy radiotherapy is given to the scar using 60kV X-rays within 24 hours of surgery. The target volume is scar plus 0.5cm margin, but if preoperative assessment shows the Keloid to be wide, very thickened, red and shiny and angry up to 1cm margin may be preferable. Careful planning and Consultant attendance & supervision of the single 10Gy dose set-up in the radiotherapy treatment room itself, together with the radiographers, is extremely important to ensure millimetre accuracy, avoiding any movement or slippage of the lead-jig when positioning the superficial X-ray unit applicator.
(2) Treatment of Unresected Keloids
Patients who have no wish for their Keloid to be excised and who are only interested in pain relief, including relief from itching and irritation, and flattening of their Keloid, then we offer, as our protocol, radiotherapy treatment every 3 months and up to 4 fractions over 1 year. In this case the applied dose every 3 months is 4Gy and either 60kV, 100kV, 160kV X-rays are used, i.e. whichever energy appropriate, bearing in mind the age of the patient. While a total dose of 16Gy in 4 fractions over 1 year may be delivered the need for all 4 fractions may not always be necessary to obtain good symptomatic relief.
We also note and make reference to Malaker et al’s protocol on the treatment of unresectable Keloids [2] and we may employ this in appropriate circumstances.
RESULTS
Our long term results demonstrate that the use of very careful planning techniques, giving only one [1] single large applied dose of 10Gy using 60KV, in the postoperative setting, and that we do achieve excellent recurrence free results as well as very acceptable cosmesis, e.g. our data for earlobe Keloid control is 100% at 4 weeks, 91.2% at 1 year and 79.4% at 5 years [1].
DISCUSSION
Consenting procedure, acute radiation effects and potential late effects & risks will also be discussed. Experience in the use electron beams will also be described but the advantages of using superficial X-rays over electrons will be demonstrated, one of which is the training of Clinical Oncologists. Many different body sites at presentation of Keloids will be illustrated, including those in patients developing post face-lift and breast reduction surgery.
We are also evaluating the use of Thermal Imaging to define Keloid margins. We already have experience in defining true extent of ‘impalpable’ Morbus Dupuytren’s in hands using this technique [3] and this will be illustrated.
CONCLUSIONS
With the use of superficial radiotherapy excellent treatment results have been produced with excised Keloids and good results with unresected Keloids. Imaging studies are underway to determine if treatment margins can be better identified and results further improved.
REFERENCES
- Treatment of Keloids by Surgical Excision and immediate Postoperative Single-fraction Radiotherapy. Ragoowansi, Raj FRCS; Cornes, Paul G. S. FRCR; Moss, Anthony L. FRCS; Glees, John P. FRCR Plastic and Reconstruct Surgery. 2003; 111: 1853-1859
- Retrospective Analysis of Treatment of Unresectable Keloids with Primary Radiation Over 25 Years. Malaker, K., Vijayraghavan, K, Hodson, I. and Al Yafi, T. Clinical Oncology (2004) 16: 290 – 298
- Pilot studies in the Application of Thermal imaging in Dupuytren’s Disease. G C Weatherburn, PhD, J P Glees MD, FRCR, Y. Qiao MSc and H. Weatherburn PhD. The Future of Medicine – The Role of Doctors in 2025. Royal Society of Medicine, 19 May 2016
Estimates of Radiation Risks Arising from the Treatment of Keloids by Radiotherapy.
Henry Weatherburn, PhD
Non-Surgical Management of Keloid Lesions
Intralesional Steroids – Efficacy and Side effects
Lennert Van Putte, MD
Role of Cryotherapy in Treatment of Bulky Keloids
Michael H. Tirgan, MD
Role of Lasers and Light-based Role of Lasers and Light-based Devices in Treatment of Keloids: When, Where and How?
Reza. F. Ghohestani, MD, PhD,
J. Gerardo Garcia, MD, A. Kate Arefnia, MD
Texas Institute of Dermatology, 24165 W IH-10,
Suite 102; San Antonio TX 78257
Director@txid.org
Introduction: Significant improvement has been made in laser technology as well as broad band light therapies for various skin diseases. We here review recent advances in laser treatment of keloids and also present our data to identity the best modality.
Goal: To determine effect of laser and light-based therapies for treatment of keloids. Methods & Results: We compared efficacy of Co2 laser with IPL and LED in 39 patients with keloids. Significant improvement in erythema noticed in majority of patients treated with IPL and LED.
Conclusion: We qualitatively conclude that laser and light-based treatment modalities may achieve favorable patient outcomes. While CO2 laser may not be an appropriate option in most cases, light-based therapies, such as LED or IPL may have a positive impact.
ABSTRACT PRESENTATION:595nm Pulsed Dye Laser for Hypetrophic and Keloid Scars Treatment. A Randomized-Controlled Study
Angela Filoni MD1, Michelangelo Vestita MD2,
Giuseppe Giudice MD2, Rossella Elia MD2,
Domenico Bonamonte MD PhD1
PURPOSE
The aim of our study was to assess the effects of the 595nm pulsed dye laser (PDL) in hypertrophic – keloid scars (aged >6 months) regardless of etiology (surgical, burns, etc).
METHODS
50 patients were recruited. The scar (at least 6 cm long) was divided into 3 equal segments (at least 2 cm long). Each segment was randomized to either treatment modality A (purpuric: 1.5ms), B (non-purpuric: 10ms), or no treatment (control). Treatment sessions were performed at day 1, 30 and 60. Fluences were delivered according to Fitzpatrick skin type.
Follow up visits were at 3 and 6 months. Standardized pictures were taken at each visit and patients were blindly assessed by a physician using the Vancouver Scar Scale (VSS).
At baseline and each visit a series of parameters (hemoglobin and melanin concentration, skin texture) were recorded by means of multispectral analysis (Antera 3DTM).
Patients were excluded if their scars were on the genitals, hands, or feet; they were < 18 year old; they had a history of light sensitivity. Written informed consent was obtained.
- Section of Dermatology, Department of Biomedical Science and Human Oncology, University of Bari, 11 Piazza Giulio Cesare, 70124 Bari, Italy
- Section of Plastic and Reconstructive Surgery, Department of Emergency and Organ Transplantation, University of Bari, 11 Piazza Giulio Cesare, 70124 Bari, Italy.
RESULTS
There was a statistically significant improvement in the mean values of the VSS in the non-purpuric group; the purpuric group showed a trend towards improvement (although not significant); the control group did not show any improvement. Hemoglobin concentration decreased significantly in the treated groups, as opposed to the control group. Skin texture varied significantly in the non-purpuric group.
DISCUSSION
Hypertrophic and keloid scars often result in patient dissatisfaction, functional impairment and decreased quality of life1. The abundance of treatments in the literature indicates that no available treatment produces a reliable improvement[1]. The PDL appears to benefit recent scars of various etiologies: improvements in erythema, pliability, thickness, and pruritus were reported in a number of studies, with only minor adverse reactions[1-3]. A recent controlled study demonstrated non-purpuric long pulse parameters to yield better results than purpuric impulses in recent scars[4]. To the best of our knowledge, this is the first study investigating the use of non-purpuric long pulse durations (>6 ms) and non-purpuric short pulse durations (0.450–1.5 ms) in aged hypertrophic and keloid scars. We demonstrated that PDL is effective in improving hypertrophic and keloid scars, and that non-purpuric pulses are superior to purpuric ones in this indication.
Contact informations:
Angela Filoni
Email: angela.filoni@gmail.com
Tel: 00393384718333
All authors declare no conflict of interest
REFERENCES
- Tziotizios C, Profyris C, Sterling J: Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics. Part II. Strategies to reduce scar formation after dermatologic procedures. J Amer Acad Derm 2012;66(1):13–24.
- Vazquez-Martinez O, Eichelmann K, Garcia-Melendez M, Miranda I, Avila-Lozano A, Vega D, Ocampo- Candiani J. Pulsed Dye Laser for Early Treatment of Scars After Dermatological Surgery. J Drugs Dermatol. 2015 Nov;14(11):1209-12.
- Cohen JL, Geronemus R. Safety and Efficacy Evaluation of Pulsed Dye Laser Treatment, CO2 Ablative Fractional Resurfacing, and Combined Treatment for Surgical Scar Clearance. J Drugs Dermatol. 2016 Nov 1;15(11):1315-1319.
- Gladsjo J, Jiang SIB. Treatment of surgical scars with the 595 nm pulsed dye laser using purpuric and nonpurpuric parameters: a comparative study. Dermatol Surg 2014;40:118–26.
CLINICAL ABSTRACT PRESENTATION: Painful Keloids. Evaluation of Risk Factors and Recommendation for Treatment.
Sofie De Schrijver, MD; Michael H. Tirgan1, MD
ABSTRACT:
Importance: Chronic painful keloids of anterior chest wall region pose a substantial impact on quality of life and functionality of some patients with keloid disorder. Given lack of optimal therapeutic tools for the underlying keloid disorder, both patients and healthcare providers often struggle in caring for these patients. Safe and effective treatments are needed for painful keloids.
Objective: To review clinical presentation and natural history of painful keloids; to explore risk factors for development of painful keloids; and to propose non- surgical intervention with intra-lesional vincristine and/or cryotherapy for control of pain.
Setting: This is a retrospective analysis of ten consecutive patients (1 male, 9 females), with chronic severe painful keloids who were seen by the author in his keloid specialty medical practice.
Intervention: Intra-lesional chemotherapy with vincristine or docetaxel, and/or topical cryotherapy was offered to all patients.
Results: There were 1 male, and 9 females, aged between 25 and 62 years old, in this cohort. Five patients had previously undergone surgery for painless anterior chest wall keloids. Nine patients had prior intralesional steroids, two had prior laser therapy and one had prior cryotherapy.
Substantial pain control was achieved in three patients with intermittent cryotherapy. Four patients had excellent pain control in response to intra-lesional vincristine, two were lost to follow-up. One patient did not pursue the proposed treatment. One patient did not response to either vincristine, cryotherapy or docetaxel.
Conclusions and Relevance: Chronic severe anterior chest wall keloid pain, was a “de novo” phenomenon in five patients and a “secondary” complication of keloid removal surgery in the other five patients. Intra-lesional vincristine successfully controlled the pain in four patients. Minimal application of topical cryotherapy was offered to three young females and controlled their pain in all three cases. Management of chronic keloid pain will have a positive impact on quality of life of patients.
Contact information
Sofie De Schrijver
Parklaan 110, 2650 Edegem, Belgium
Conflict of Interest: None
Funding: None
IRB Assessment: The research project was determined by Western IRB to meet the conditions for exemption under 45 CFR 46.101(b)(4).
Keywords: Pain, Painful, Keloid, Cryotherapy, Chemotherapy
Abbreviations: Keloid Disorder (KD)
Steroid Tape as an Adjunct in the Management of Keloid and Hypertrophic Scars
Mr Ioannis Goutos, FRCSEd(Plast), MSc;
Prof Rei Ogawa
INTRODUCTION
The percutaneous delivery of steroids to keloid lesions and hypertrophic scars was first described in the dermatological literature in the 1960s and currently represents on of the mainstays of specialist scar management protocols in the Orient. The is paucity of systematic reviews or synthetic summary reports in the international literature appraising the evidence base for the adoption of steroid tape in clinical practice.
METHODS
A detailed English and Japanese literature review was performed using the Pubmed and the Japan Medical Abstracts Society database with the following keywords: steroid; tape; flurandrenolone and flurandrenolide. Reports were stratified using the Joanna Briggs Institute Levels of Evidence and data were extracted relating to the maximum dose of steroid that can be delivered safely, the reported therapeutic efficacy, as well as the side effects associated with the percutaneous delivery of steroids.
RESULTS
Eighteen English language literature and two Japanese relevant manuscripts were identified with the majority representing level 4 evidence. The conclusion from our study is that steroid tape has the potential to be a safe and patient-friendly adjunct to scar management for carefully selected cases of keloid and hypertrophic lesions. The main limitation for its widespread adoption is the lack of data to enable the determination of safe exposure thresholds in adult and paediatric patients.
CONCLUSION
Despite the existing encouraging reports regarding the potential to be a useful adjunct in scar management, steroid tape is not widely used apart from a limited number of scar services worldwide. Further research is warranted to delineate the role of this modality in specialist scar management protocols.
Disclosures: None
Contact information
Mr Ioannis Goutos, FRCSEd(Plast), MSc Burn Care
Centre for Cutaneous Research, Blizard Institute
4 Newark Street, London, E1 2AT
ioannisgoutos@hotmail.com
Prof Rei Ogawa
Department of Plastic, Reconstructive and Aesthetic
Surgery, Nippon Medical School, Tokyo, Japan
prs.ogawa@gmail.com
Keloidal Morphea : Report of An Atypical Case with Satisfying Outcome
Hammami F1, Bahloul E1, Frikha F1, Masmoudi A1,
Boudaya S1, Mseddi M1, Turki H1
INTRODUCTION
Keloidal morphea is a very rare form of scleroderma that may occur with localized or systemic scleroderma (SS). We hereby report a new case of keloidal morphea with remarkable findings.
CASE REPORT
A 52-year-old female patient with a long history of rheumatoid arthritis had been followed up in our department for localized scleroderma morphea type. After 11 years of follow up, she developed firm erythematous slightly itchy nodules on the trunk. These keloidal lesions initially started on the site of a skin biopsy and gradually spread to involve the rest of preexisting morphea plaques (figure). There was no clinical sign of SS. Laboratory analyses showed a positive ANA (1/640) with negative tests for anti-Scl-70 and anti-centromere. The skin biopsy performed on a keloidal lesion revealed a thinned epidermis with the presence of bundles of thick collagens in the dermis.
Based on the these clinical and histopathological findings, the diagnosis of keloidal morphea in the context of localized scleroderma was thus made. Our patient had been treated with several medications including: Topical corticosteroids and PUVA therapy but it could not achieve satisfactory results. After 10 years of follow up, she developed recurrent attacks of abdominal pain with diarrhea and small amount of mucous and bloody discharge. The colonoscopy revealed patchy erythematous areas with superficial ulcers.
Colonic biopsies confirmed the presence of severe transmural inflammatory infiltrate with eosinophils and evidence of an epithelioid granuloma. She was diagnosed with Crohn’s disease and was treated with Sulfasalazine 3 g daily. After 12 months of treatment, while intestinal symptoms remained stable, we have noticed clinical improvement of her scleroderma plaques.
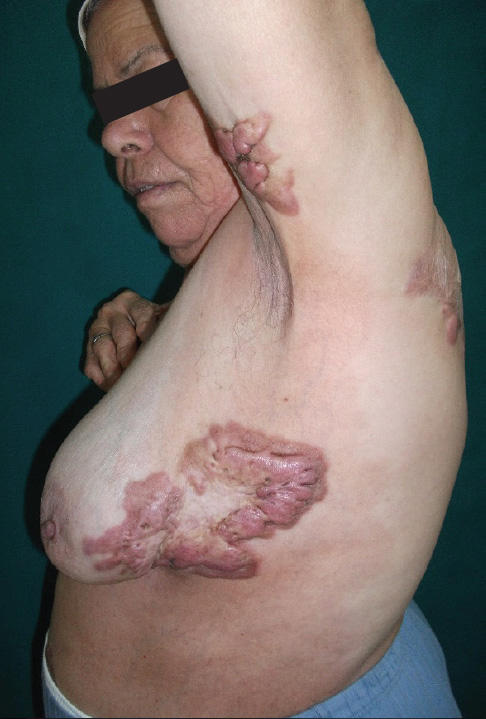
DISCUSSION
In this case, we report three remarkable findings. The first is that keloidal morphea is a rare disease and has been previously described in the literature with less than fifty cases. In a recent literature review of previously reported cases, clinical data of 43 patients were presented. The median age was 41 years (3-70). The lesions affected with preference the trunk, as in case of our patient. Unlike our patient, the majority of patients presented with sclerodactyly as well as extra cutaneous manifestations of SS.
The second remarkable finding in our case, is that keloidal morphea is associated to rheumatoid arthritis and Crohn’s disease. In a previous study reporting 472 patients with localized scleroderma, association between morphea and rheumatoid arthritis and inflammatory bowel disease was reported each one in 0.2% of cases. This association is suggestive for a common pathogenetic autoimmune background.
The last remarkable finding is the improvement with Sulfasalazine. There is no consensus for the treatment of choice for keloidal morphea. Several treatment modalities have been tried including corticosteroid, topical vitamin D analog, D-penicillamine, PUVA and methotrexate. However, these treatments have shown unsatisfying results. To the best of our knowledge, an improvement induced by Sulfasalazine has never been reported. It could be related to its anti-inflammatory action.
CONCLUSION
Keloidal morphea is a rare condition. The pathogenesis of this disease is still unclear. Association with rheumatoid arthritis and Crohn’s disease is suggestive of a common pathogenetic autoimmune background. The successful outcome with Sulfasalazine suggests that this drug may be useful in future cases.
Contact Information
Emna Bahloul, MD
Address: Department of Dermatology
Hedi Chaker Hospital 3029 Sfax Tunisia
E-mail: emnabahloul86@gmail.com
Telephone number: +21692020209
Fax number: 00216 74 24 26 27- 00216 74 24 45 11
CLINICAL CASE PRESENTATION:
Earlobe Keloid Regression by Bottom Ligature and Injection with Corticosteroid and 5-FU
Tian Ming, Dong JiaoYun, XiQiao Wang
Burn Centre, Ruijin Hospital, Jiaotong University
Medical School, 197 Ruijin Road, Shanghai,China
A 34-year-old girl suffered from earlobe keloid for 6 years after puncture of ear ring. 2 years ago, she received a corticosteroid injection for 6 times, it had a little reduction. After 6 months, it recurred with more larger size about 1.5 × 1.5 × 1.2 cm. This time, we use the silk ligatured at the bottom of keloid, persisted for 23–24hs each day , and injected with corticosteroid and 5-FU each month at the bottom and entity. After 4 months, the bottom became more and more thin, and the entity became purple and then undergo drying necrosis , 1 month later, the necrotic entity fall off at the bottom, almost without wound. Thereafter, treated with 3 injections, the keloid completely resolved.
Conclusion: The bottom ligature could reduce the blood supply, cause the entity necrosis, and accelerate the keloid regression on the base of injection of corticosteroid and 5-Fu.
Clinical Research / Abstract Presentations
Optimization of Surgical Management for the Treatment of Auricular Keloids
Tae Hwan Park., MD, PhD
Department of Plastic and Reconstructive Surgery,
CHA Bundang Medical Center, CHA University,
Songnam, Republic of Korea
ABSTRACT
Keloids are often resistant to treatment with high recurrence rates. Ears are one of the most commonly involved anatomical areas and auricular keloids usually occurs following ear piercing with an incidence of 2.5%. Their cosmetic deformity and psychological trauma are very troublesome to the patients. In my opinion, surgical excision followed by adjuvant therapy is gold standard for auricular keloids in Asian population. In terms of surgical management of keloids, balancing between free surgical margins and minimizing tension is crucial to prevent keloid recurrence. In addition, anatomical locations in ears and planned postoperative adjuvant therapy should be carefully considered to provide better outcomes for the treatment of auricular keloids.
Contact information:
Tae Hwan Park., MD, PhD
Department of Plastic and Reconstructive Surgery
CHA University College of Medicine
59 Yatap-ro, Bundang-gu, Seongnam,
Gyeonggi 13496, Korea
Tel: +1-240-205-2650
E-mail: hard-piano@hanmail.net
Conflict of Interest: None.
Financial Disclosures: None.
Ethical Approval
This study was approved by the institutional review board of the CHA University and conducted in accordance with the Declaration of Helsinki.
The Effects of Post-Operative Intralesional Corticosteroids in the Prevention of Recurrent Earlobe Keloids: A Multispecialty Retrospective Review
Lamont Jones, MD, MBA
Hypo-Fractionated Electron Beam Radiation Therapy, A Retrospective Analysis of 568 Keloid Patient with 834 Keloid Lesions
Xiao Long, MD
We aimed to analyze the outcomes of hypofractionated high-energy electron beam radiotherapy for the treatment of keloids. From February 1998 to January 2012, 568 patients with a total of 834 keloids underwent radiotherapy: 826 lesions with postoperative radiotherapy, and 36 with skin-grafting. Lesion size was >5 cm in 335 keloids. An electron-beam of 6 or 7 MeV was used, with a total dose of 18 Gy (two fractions with a 1-week interval) covering the lesion with a 1-cm margin. The time between surgery and radiotherapy was 24-48 h. Skin-grafted patients underwent radiotherapy 10-15 days after the operation. The median follow-up was 40 months (range: 12-160 months). The local control rate was 88.25% (736/834). The relapse rate was 9.59% (80/834), and the time to relapse was 6-28 months (median: 12 months). Univariate analyses showed that gender, age, keloid size, keloid site, skin grafting, and operation-to-irradiation interval influenced the local control rate. Multivariate analysis showed that the relapse rate was correlated with gender (P = 0.048), age (P < 0.01), operation-to-irradiation interval (P < 0.01), keloid site (P < 0.01), surgical method (P = 0.04) and keloid size (P < 0.02). Adverse effects were observed in 9.83% (82/834). No radiationinduced cancers were observed. Hypofractionated high-energy electron beam radiotherapy for keloids yielded excellent outcomes, especially in cases without skin grafting. Early postoperative radiotherapy with limited hypofractionation could be a good choice for keloid treatment.
Keywords: assessment; electron beam; keloids; prognosis; radiotherapy
Research in Post Sternotomy Keloids
Frank Niessen, MD, PhD
Treating Keloids with Adjuvant High-Dose Rate Brachytherapy
Jonathan Tsao MD, Sarah Rauth MD,
Jasper Yuen MD, Kailin Lawrence MRTT,
Gwen Bond RN, Eric Sabondjian CCPM
ABSTRACT
The literature supports the role of postoperative adjuvant radiotherapy for keloids in decreasing the risk of recurrence. External beam radiation has usually been employed but more recently described is the use of brachytherapy. This institutional retrospective review analyzed the clinical results for 26 patients with 36 keloids treated over the last 3 years with surgical excision and postoperative HDR brachytherapy. The patients ranged in age from 19 to 78 years and there were 7 males and 19 females. Eighteen were of Asian or African race. All patients were treated to a dose of 18 Gy in 3 fractions at a depth of 0.5 cm. within 36 hours following excision. Median follow up was 11 months and median active treatment length was 7.6 cm. There was no case of recurrence but there were 3 cases of transient hyperpigmentation, 4 cases of wound dehiscence, and one case of infection. These early clinical results are promising but longer follow up is required to better evaluate the full potential of adjuvant HDR brachytherapy for the treatment of keloids.
Topic Code: Brachytherapy, Keloids
Contact Information
Jonathan Tsao MD
Division of Radiation Oncology, Carlo Fidani Peel
Regional Cancer Centre, Trillium Health Partners,
2200 Eglinton Avenue West, Mississauga, ON
Canada L5M 2N1
Tel: (905) 813 1100 x4803 | Fax: (905) 813 3962
Email: jonathan.tsao@thp.ca
Brachytherapy as an Adjunct in the Management of Keloid Lesions
Mr Ioannis Goutos, FRCSEd(Plast), MSc;
Prof Rei Ogawa
INTRODUCTION
Radiation therapy is a well-recognised modality for the adjuvant treatment of keloid scars and is conventionally delivered as external beam (EBRT) using a large apparatus at a distance from the lesion. Brachytherapy employs specialised equipment to enable the delivery of treatment in the immediate vicinity of the keloidal tissue and has a number of advantages over EBRT.
METHODS
An English literature review was performed with keywords ‘brachytherapy’ and ‘keloid’ using the databases PubMed, Embase and Web of Science from their individual dates of inception until June 2017. Studies pertinent to the field are presented ina chronological manner to depict the evolution of different brachytherapy strategies over the last decades. We also reviewed the literature with regards to the risk of secondary carcinogenesis, which are relevant to shared decision-making in the clinical setting of keloid practice.
RESULTS
We identified seventeen case series of relevant articles [four pertinent to low dose rate interstitial, eight to high dose rate (HDR) interstitial and five to HDR superficial brachytherapy] as well as one systematic review and one meta-analysis appraising this modality in the management of keloid lesions. Low dose rate interstitial brachytherapy was first introduced in the English literature in 1976 and currently appears to have been superseded by the high dose rate interstitial variant; the latter compares favourably to more traditional modes of radiotherapy in terms of recurrence as well as rates of symptomatic relief from keloidal symptoms. Superficial brachytherapy was introduced more recently in the literature and appears to be associated with favourable therapeutic outcomes compared to external beam radiation therapy.
CONCLUSION
Brachytherapy is a valid modality of radiotherapy for the adjuvant treatment of keloid scars, with high dose rate interstitial and surface regimens gaining in popularity over recent years. No reports of secondary carcinogenesis have so far been reported using this type of radiotherapy treatment. Further research needs to focus on randomised controlled trials to further establish the role of different radiotherapy modalities in keloid scar management.
Disclosures: None
Contact information
Mr Ioannis Goutos, FRCSEd(Plast), MSc
Burn Care Centre for Cutaneous Research,
Blizard Institute
4 Newark Street, London, E1 2AT,
ioannisgoutos@hotmail.com
Prof Rei Ogawa
Department of Plastic, Reconstructive and Aesthetic
Surgery, Nippon Medical School, Tokyo, Japan
prs.ogawa@gmail.com
AURICULAR KELOID MANAGEMENT:
Clinical Outcome of Intralesional Excision and Postoperative Triamcinolone Acetonide Intralesional Injection
Young-Jun Choi, MD, Jung Yup Kim, MD,
Jae-Hui Nam, MD, Ga-Young Lee, MD, PhD,
and Won-Serk Kim, MD, PhD
Department of Dermatology, Kangbuk Samsung
Hospital, Sungkyunkwan University School of
Medicine, Seoul, Republic of Korea
ABSTRACT
Background: Various treatments such as surgical excision, steroid injection, pressure therapy, and Radiation Therapy (RT) are available for auricular keloid. In particular, surgical excision in the treatment of auricular keloid is important, because recurrence rates are low compared to other sites.
Objectives: We aimed to evaluate the clinical outcome of intralesional excision followed by postoperative Triamcinolone Acetonide Intralesional Injection (TA ILI) in the management of auricular keloid.
Methods: We conducted a surgery records and charts review of patients who underwent auricular keloid management with intralesional excision and TA ILI. We also analyzed the recurrence rate over a 2-year period and evaluated the patient satisfaction using 11-point questionnaire (0-10).
Results: A total of 18 Korean patients (2 males and 16 females), mean age of 26.5 years, with total 20 lesions were evaluated (2 patients had bilateral lesions). Lobular type (n = 10, 50%) was the most common, followed by anterior/posterior button (n = 3, 15%), wrap-around (n = 3, 15%), dumbbell (n = 2, 10%), and sessile type (n = 2, 10%). Total recurrence rate was 5% (1 of 20) within 24 months follow-up period, and mean satisfaction score was 9.6 (more than moderately satisfied). No serious and persistent adverse events were reported during the follow-up period.
Conclusion: We confirmed that TA ILI after intralesional excision can be effective for auricular keloid management. With an effective surgical procedure and minimal postoperative treatment, a low recurrence rate close to that of postoperative RT was obtained.
Keywords: Auricle, ear, keloid, intralesional excision, intralesional injection, triamcinolone acetonide
THE KELOIDS BANE, WITHOUT THE PAIN – A New Approach in the Treatment of Keloids: Tixel-Associated Topical Triamcinolone Acetonide and 5-Fluorouracil Delivery
Ofir Artzi, MD, J. Merhabi Friedman O
ABSTRACT
Background and objectives: Keloids are challenging to treat due to their inadequate response and high recurrence rate. Intralesional Triamcinolone Acetonide (TAC) injection with or without 5-fluorouracil (5FU) is considered first-line treatment for keloids. Three significant disadvantages of intralesional injections are pain associated with the procedure, the uneven topography and epidermal atrophy. Fractionated ablative carbon dioxide (CO2) Laser- Assisted Drug Delivery (LADD) of topical solution has been described for many indications, including scar remodeling. The study examined a novel non-laser, non-painful fractional thermal resurfacing system (Tixel, Novoxel) which can generate ablative as well as non-ablative micropores permeating the skin for transdermal delivery of a topical solution containing TAC and 5-FU in the treatment of Keloids.
Materials and Methods: 32 keloids in 12 patients (5 males, 7 females. 6 Adults and 6 Children <12) were treated with the device (Setting: 5-8 ms, 500- 1000 protrusion, single pulse) followed immediately by TAC and 5FU application. Sonophoresis was performed to enhance drug penetration (Impact, Alma lasers, Frequency: 50 Hz, Intensity 50%, 5 min). All keloids received 8 treatments, 2-3 weeks apart. Photographs were taken at baseline and 1 month following the last treatment. Treatment outcomes were evaluated using the Vancouver Scar Scale (VSS). In addition, 4 parameters (Toughness, Thickness, Color and general aesthetic impression) were scored at base line and 1 month post final treatment, using a quartile grading scale (0-normal skin, 4-worst scar). Pain levels and participant or parent satisfaction were assessed by the patient using the Visual Analog Scale (VAS) and quartile grading scale (0-not satisfied, 4-highly satisfied) respectively.
Results: Mean keloid VSS reduced from 8.69 ± 1.75 to 4.32 ± 1.42 post 8 treatments. Mean reduction of toughness, thickness, color and general aesthetic impression were reduced as following: 3.1 ± 0.43 → 2.2 ± 0.31, 3.4 ± 0.5 → 1.9 ± 0.63, 2.7 ± 0.21 → 2.4 ± 0.25, 3.23 ± 0.44 → 1.6 ± 0.64, respectively. Mean treatment pain VAS score was 2.1 ± 1.62. Patients rated their satisfaction level as “moderate-high.” No severe adverse reactions were noted.
Conclusion: This combined approach using a nonlaser, non-painful novel drug delivery system of TAC and 5-FU, can be a promising modality for the treatment of keloids, especially in pediatric patients.
Contact Information:
Ofir Artzi, MD
20, Lilinblum st Tel Aviv, 65132
benofir@gmail.com
Basic Science Research / Abstract Presentation
The MicroRNA Methylome Landscape in Keloid Pathogenesis
Lamont R. Jones, Joana Kam, Kang Mei Chen,
Josena Stephen, Laura Rodriguez-Garcia,
Indrani Datta, George Divine, Maria J. Worsham
LEARNING OBJECTIVES
- At the conclusion of this activity, the participant should be able to: Discuss how miRNA impacts gene expression and contribute to disease states.
- At the conclusion of this activity, the participant should be able to: Discuss the role of miRNA in the pathogenesis of keloids.
Study Objective: Identify miRNA contribution to keloid pathogenesis
Design: Prospective Cohort
Methods: Sixteen fresh keloid with paired normal fresh tissue samples, from the head and neck area, were analyzed using the Illuminal Methylation EPIC Chip®. The platform analyzes 880k CpGs of which 842,625 were carried forward after quality control and data cleanup. Of the 842,625 CpGs, 6763 fell into genomic region of microRNAs and were retained for statistical analysis. A paired t-test was used among keloid vs normal to calculate raw p-values and then FDR corrected p-values on the 6763 CpGs. There were 2 pairs of duplicate samples from the same patient which were resolved by taking the average of methylated and unmethylated intensities for each pair which resulted in 15 paired samples for analysis. Statistical analyses was performed with conversion of methylated and unmethylated intensities to M-values (formula from Du et.al, Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis.
BMC Bioinformatics 2010). Statistically significant results were divided into 3 tiers (tier1 with adaptive FDR ≤ 0.05, tier2 with tier1 threshold along with keloid vs normal BetaRatio ≥ 2 or keloid vs normal BetaRatio ≤ 0.50, tier 3 with tier 1 & tier 2 threshold along with absolute difference keloid minus normal AvgBeta = 0.2). Hyper and hypo status of a CpG site is based on keloid vs normal BetaRatio < 1 (i.e hypo) and keloid vs normal BetaRatio ≥ 1 (i.e hyper). There were 1836 CpGs in Tier1 at FDR ≤ 0.05 level, which were in genomic region(s) of 649 microRNA genes. Three of the microRNAs were in Tier 2 and none made it to Tier 3. Because miRNAs perform their important functions via their target genes, the Tier1 miRNAs were used to extract coding gene (mRNA) targets in Ingenuity’s IPA knowledgebase. This IPA resource draws from online databases such as TargetScan, TarBase, miRecords, and Ingenuity Expert Findings for prediction of binding sites and target genes of miRNAs. The degree of confidence that a target gene is associated with a miRNA is characterized in these databases as either “high” (predicted) or “experimentally observed” (validated).
Conclusion: There were 649 Tier1 microRNAs and 3 Tier2 miRNA (miRNA 548W, 548N and127`3E. None of the Tier2 miRNAs had target genes in IPA. Among the 649 Tier1 miRNAs, 5 miRNAs: mir-147a, 203a-3p, 292-3p, 486-5p, and 499-5p have 12 coding genes (mRNA) targets: VEGFA, ABL1, RUNX2, SOCS3, TP63, AR, AFF3, ARID4B, FOXO1, PTEN, TWF1 and SOX6, which were indicated as experimentally validated in IPA. Characterization of biological pathways enriched by these target genes to support the 5 miRNAs as candidate miRNAs in keloid pathogenesis, is underway.
Can Anomalies of the Atypical Chemokine Receptor 1 (ACKR1) Gene Explain the Dysregulated Microenvironment of Keloids and Some Aggressive Breast Cancer Subtypes?
Haythem Y. Ali1, Melissa Davis2, Brittany Jenkins3, Edward Walton4, Eleanor M. Walker5, Lamont Jones6
Keloids are benign fibroproliferative tumors which may develop after skin injury in susceptible individuals. Genomic studies indicate dysregulation of genes important for apoptosis, extracellular matrix formation, and immunity. The importance of the composition of tissue microenvironments in influencing and promoting disease states impacted by inflammation is clearly demonstrated.
Various studies have explored the influence of the cancer microenvironment on carcinogenesis. The microenvironment is shown to exert pro-malignant properties, depending on its composition resulting in tumor growth and progression.
Because keloids disproportionately affect African American patients it may serve as a model to better understand how their tissue microenvironment, including the genetic background which drives Keloid formation, predisposes them to certain immunogenic cancers such as triple negative breast cancer and inflammatory breast disease. Our research team is interested in uncovering the genetic underpinnings of these disparities and to determining if this keloid predisposition is somehow related to incidence of breast cancer and/or tumor phenotypes.
We hypothesize that the altered immune response in keloid development may also be genetically linked to tumor immune response, particularly in women of African descent. Recently, we have identified an association between an African-specific allele of the Atypical Chemokine Receptor 1 gene (ACKR1) and breast tumor immune response. We are investigating if this gene would also predict keloid status/ predisposition. For this study, we are currently building an investigational cohort of breast cancer patients, tracking the co-occurrence of breast cancer and keloid diagnosis, in our Henry Ford Health System electronic medical record database. So far, out of the breast cancer diagnoses between 2010-2017, over 200 have also been diagnosed with keloids. Over 90% of these co-occurrences are African American, and of all AA breast cancer cases nearly 60% have keloids. This suggests that AA women who are diagnosed with breast cancer do also have a predisposition for keloids. We have begun genotyping the ACKR1 allele in a subset of AA and Caucasian American keloid cases and are also investigating the expression of ACKR1 in keloid tissues using IHC. We will present our findings related to the associations of ACKR1 keloid expression with the ACKR1 African-specific allele as well as ACKR1 expression in both keloid and breast cancer tumor for co-occurrences. As a secondary analysis, we will also investigate correlations of immune responses in breast tumors and keloids, related to ACKR1 expression.
- Henry Ford Hospital, Department of Internal Medicine, Division of hematology and Oncology: presenting First Author.
- Henry Ford Hospital, Department of public health sciences:Co-first Author.
- Henry Ford Hospital, Department of public health sciences.
- Wayne State University.
- Henry Ford Hospital, Department of Radiation Oncology.
- Henry Ford Hospital, Department of Otolaryngology.Senior Author
Canvassing Tissue Microenvironments for Immune Disease Markers as Potential Candidates of Immunotherapy Responsiveness: Applicability to Keloid Pathogenesis
Maria J Worsham, PhD, FACMG
Objectives: The T-cell repertoire reflects the host immune response to the disease process. In keloid disease, a common fibroproliferative disorder of unknown etiology, increased numbers of T cells and macrophages are thought to contribute to its pathogenesis. However, while a link between immune responses is well known for other fibrotic disorders and in malignancy, it remains undetermined in keloids. The Cancer Genome Atlas (TCGA) has profoundly illuminated the genomic landscape of human malignancy. Genomic and transcriptomic data derived from bulk tumor samples have been used to study the tumor microenvironment, and measures of immune infiltration define molecular subtypes of various cancers. However, we know little about how changes in non-malignant fibroproliferative immune microenvironments contribute to disease initiation, progression, and recurrence. Our studies, aimed at identifying immune phenotypes or immune signatures predictive of progression to breast cancer (BC) from benign breast disease (BBD), is aimed at immuno-prevention of progression of premalignant BBD lesions to BC. In squamous head and neck cancer (HNSCC), our studies are directed at developing novel biomarkers for responsiveness to immune checkpoint therapy. Our purpose here is to illustrate the feasibility of next generation (NGS) approaches of RNA sequencing (RNAseq) to identify distinct immune cell profiles that are easily extrapolated to keloid tissue for identifying potential keloidderived immune biomarkers for consideration in the development of immune-related treatments that can inhibit and circumvent the fibroproliferative disease process.
Methods: Immune microenvironment landscapes in women with BBD who progress to breast cancer (BBD cases) and those that do not (BBD controls) and HNSCC tumors and normal controls were characterized using RNAseq as opposed to laborious and restrictive immunohistochemistry approaches. The Oncomine™ Immune Response Research Assay (OIRRA, ThermoFisher) enables the characterization of gene expression levels from approximately 395 immune-related genes across 36 functional annotation groups. The relative immune cell infiltration levels in disease tissue are computed by an over-representation score (ssGSEA) from the normalized RPM (relative parts per million) levels of signature genes from 36 immune related functional groups. For consensus cluster analysis, Euclidean distances were calculated and hierarchical clustering was performed using Ward’s D2 method.
Results: Our pilot BBD studies identified several significant differential immune clusters and differentially expressed immune genes between BBD cases and BBD controls. The proliferation cluster showed the most distinct difference between BBD cases and BBD controls. In HNSCC, consensus cluster analysis revealed 2 significantly differentiated clusters (p = 0.002). Cluster 1 contained 90% tumors (8/9) and 100% of cluster 2 is normal tissue only. With false discovery rate adjusted p-values < 0.05, relative to the normal tissue immune cluster 2, the tumor cluster 1 is characterized by up regulation of PD-1 signaling, dendritic cells, antigen presentation, myeloid cells, B-cells, and leukocyte migration and down regulation of macrophages and type 1 interferon signaling.
Conclusion: Emergence of a highly significant proliferative immune cluster in BBD cases when compared to BBD controls is suggestive of microenvironment contributory factors that likely support the increase risk for progression to BC in BBD women with proliferative BBD as compared to non-proliferative BBD. In HNSCC, up regulation of tumor immune phenotypes of PD-1 signaling and myeloid cells and down regulation of macrophages and type 1 interferon signaling, suggestive of immune suppression, and of dendritic cells and antigen presentation, suggestive of immune stimulation, underscore that the ultimate fate of cellular immune responses is determined by the balance between negative and positive signals delivered by costimulatory molecules to T cells. In keloids, understanding the contribution of the immune microenvironment would provide much needed insights into how targeting the inflammatory phase, this crucial first phase of wound healing, can modulate the outcome of the healing response to achieve inhibition and/or circumvention of fibroproliferative disease processes.
Admixture Mapping and Increased Risk Factors for Fibrosis in African Ancestry Populations
Shirley Brody Russell, PhD
ABSTRACT
Admixture mapping identifies gene variants involved in ethnic variation in disease risk and/or severity. It is based on the idea that the different prevalence of a genetic disease in different populations is due to differences in frequency of predisposing gene variants. In admixed populations these variants are expected to occur more often in chromosomal regions inherited from the ancestral population with the higher frequency of the disease. The approximate 20-fold increase in prevalence observed for keloid formation in African Americans relative to Caucasians in the US makes admixture mapping a reasonable and costeffective approach to identify chromosomal regions containing genes that promote keloid formation. We have conducted admixture mapping and whole-exome association using 478 African Americans (AA) samples (122 cases, 356 controls) with exome genotyping data to identify regions where local ancestry associated with keloid risk[1]. A significant mapping peak was observed on chr15q21.2–22.3. This peak included NEDD4, a gene previously implicated in a keloid genome-wide association study (GWAS) of Japanese individuals[2] later validated in a Chinese cohort[3]. While we observed modest evidence for association with NEDD4, a more significant association was observed at MYO1E and ADAM10. After doing fine mapping of high priority genes in this region, including introns, exons, and 20K upstream and downstream elements, and subjecting the data to single variant, whole gene, and PrediXcan analysis, MYO1E remained among the most significantly associated genes. We are examining biological relevance of candidate genes by testing effects of silencing and overexpression on an in vitro keloid phenotype, and on cell growth and migration. We have observed no effects of silencing MYO1E, NEDD4, and ADAM10, on expression of the differentially expressed genes, dermatopontin, SFRP1, MMP1, JAG1 and IGFBP5 in keloid fibroblasts. However, silencing MYO1E and NEDD4, but not ADAM10 decreased growth of normal and keloid fibroblasts. We have begun to examine the effects of overexpression of MYO1E and NEDD4 and are initiating validation studies of our sequencing findings using samples from an independent Nigerian population provided by Ernst Reichenberger.
Keloid formation is only one of a group of fibroproliferative diseases characterized by an exaggerated response to injury that occur at higher frequency or with more severe manifestation in people of African ancestry. These diseases include hypertension, nephrosclerosis, scleroderma, sarcoidosis, allergic disease, and uterine fibroids. It may be that a common etiopathology operates in fibroproliferative diseases, and that common genetic factors may account for their unusual racial distribution. Differential selection on genetic variants in ancestral environments that coincidentally predispose to disease can be an underlying cause of these unequal prevalence patterns. Selected genes may be pleiotropic, affecting multiple phenotypes and resulting in more than one disease or trait. We have proposed that the increased prevalence of a subset of fibroproliferative disorders in individuals of African ancestry is due to selection for an enhanced Th2 response that confers resistance to helminthic infections,and concurrently increases susceptibility to fibrosis due to the profibrotic action of Th2 cytokines, and have provided evidence that adaptation of the immune system has shaped the genetic structure of these human populations in ways that alter the distribution of multiple fibroproliferative diseases[4]. We have recently constructed a genetic risk score (GRS) of fibroproliferative disease riskincreasing alleles using 147 linkage disequilibriumpruned variants identified through genome-wide association studies of seven fibroproliferative diseases with large African-European prevalence disparities. A comparison of the fibroproliferative disease GRS between 1000 Genomes Phase 3 populations detected a higher mean GRS in AFR (mean = 148 risk alleles) than EUR (mean =136 risk alleles; T-test p-value = 1.75 × 10-123 )[5]. To test whether differences in GRS burden are systematic and may be due to selection, we employed the quantitative trait loci (QTL) sign test. The QTL sign test result indicates that population differences in risk-increasing allele burdens at these fibroproliferative disease variants are systematic and support a model featuring selective pressure (p value = 0.011). These observations were replicated in an independent sample and were more statistically significant (T-test p-value = 7.26 × 10-237 , sign test p-value = 0.015). This evidence further supports the role of selective pressure acting to increase frequency of fibroproliferative alleles in populations of African relative to European ancestry populations.
REFERENCES
- Velez Edwards DR, Tsosie KS, Williams SM, Edwards TL, Russell SB. Admixture mapping identifies a locus at 15q21.2-22.3 associated with keloid formation in African Americans. Hum Genet. 2014;133(12):1513- 23. Epub 2014/10/05. doi: 10.1007/s00439-014- 1490-9. PubMed PMID: 25280642.
- Nakashima M, Chung S, Takahashi A, Kamatani N, Kawaguchi T, Tsunoda T, et al. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genet. 2010;42(9):768-71. Epub 2010/08/17. doi: 10.1038/ ng.645. PubMed PMID: 20711176.
- Zhu F, Wu B, Li P, Wang J, Tang H, Liu Y, et al. Association study confirmed susceptibility loci with keloid in the Chinese Han population. PLoS One. 2013;8(5):e62377. Epub 2013/05/15. doi: 10.1371/ journal.pone.0062377. PubMed PMID: 23667473; PubMed Central PMCID: PMCPMC3646817.
- Russell SB, Smith JC, Huang M, Trupin JS, Williams SM. Pleiotropic Effects of Immune Responses Explain Variation in the Prevalence of Fibroproliferative Diseases. PLoS genetics. 2015;11(11):e1005568. Epub 2015/11/06. doi: 10.1371/journal.pgen.1005568. PubMed PMID: 26540410; PubMed Central PMCID: PMCPMC4634921.
- Hellwege JN, Torstenson ES, Russell SB, Edwards TL, Velez Edwards DR. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12(8):e0182791. Epub 2017/08/10. doi: 10.1371/journal.pone.0182791. PubMed PMID: 28792542; PubMed Central PMCID: PMCPMC5549739.
A Comparison of Apoptosis Levels in Keloid Tissue, Physiological Scars and Normal Skin
Xiao Long, MD
Apoptosis is a process of programmed cell death that occurs in multicellular organisms. The mitochondrial pathway plays a paramount role in apoptosis. In this study, the expression levels of key factors in the mitochondrial pathway and the cell proliferation factor (PCNA) were measured to evaluate the level of apoptosis and proliferation in keloid scars, physiological scars and normal skin tissue. Thirty samples were taken from 30 patients: 10 keloid patients, 10 physiological scar patients and 10 patients without obvious scarring. All 30 patients were selected randomly from the Department of Plastic Surgery at Peking Union Medical College Hospital from June 2016 to December 2016. Hematoxylin and eosin staining and Masson staining were used to observe the differences in histology and fiber tissue content. Mitochondrial pathway factors (caspase-3, caspase-8, caspase-9, Bcl- 2, Bax, cytochrome-c) and PCNA expression levels were detected by immunohistochemistry and were analyzed as the percentage of positively stained cells in the epidermis and dermis. Relative protein expression levels were measured by western blotting. Compared with physiological scars and normal skin tissue, keloid tissue had an increase in fiber number and decrease in cell content. In our immunohistochemical and western blot analyses, all tissue types showed similar expression levels of the mitochondrial pathway factors. However, the percentage of PCNA-positive cells and the relative protein expression level of PCNA were significantly higher in keloid tissue. Keloid has a similar apoptosis level as physiological scars and normal skin but has a higher expression of PCNA, indicating that keloid scars have high levels of proliferation and normal apoptosis.
Keywords: Keloid; apoptosis; physiological scar; proliferation; skin
Micro/Nanotechnology for Treatment and Diagnosis of Abnormal Scar (Keloid and Hypertrophic Scar)
Chenjie Xu, PhD1,2,*
ABSTRACT
Abnormal scars result from over-exuberant wound healing and cause significant pain, impair mobility, and psychological anguish. Current standard of care is inadequate and lack of acceptable therapeutics and diagnostics. Molecular diagnostics are favourable in identifying high-risk wounds before maturity. To date, abnormal scars are diagnosed through symptomatic and visual appearance. Molecular diagnostics can provide clinicians with better information to make good clinical decision and early interventions, and to monitor the treatment progress. On the other hand, therapeutics are dissatisfactory due to: lack of efficacy, patient experiencing pain and involvement of healthcare personnel. Developing self-applied therapeutics minimizes healthcare personnel involvement and reduces overall healthcare burden. Dr. Chenjie Xu’s laboratory is dedicated to address these challenges by using the latest development in micro/nanotechnologies. To faciliate the diagnosis and prediction, his team developed two kinds of molecular sensors. The first one is a near-infrared (NIR) fluorescence activatable molecular probe for specific detection of keloid-derived fibroblasts (KF). These probes can turn on its NIR fluorescence in the presence of fibroblast activation protein-alpha (FAPα), which is overexpressed in activated keloid fibroblasts compared to normal fibroblasts. The second one is a transdermal nanoparticle that can recognize the abnormal expression of cellular connective tissue growth factor (CTGF). During cell culture, both sensors displayed sufficiently high resolution to distinguish hypertrophic and keloidal fibroblasts from normal fibroblasts, including the regulatory effect of transforming growth factor (TGF)-β agonists and TGF-β antagonists. To evaluate clinical feasibility, they were applied topically to the skin of live mice and rabbits, as well as ex vivo human skin models. They demonstrated transepidermal penetration, and the ability to visually and spectroscopically identify and quantify the underlying abnormal fibroblasts. Finally, we developed a self-administered microneedle device based on drug-free physical contact for inhibiting abnormal scars. Its therapeutic activity through microneedle contact eliminates hazards associated with toxic anti-scarring drugs while self-treatment enables flexibility in administration.
The device was first tested on (keloid) fibroblasts to ascertain its ability to inhibit the proliferation of fibroblasts. Later the microneedle patch was examined on the rabbit ear hypertrophic scar model to explore its potential in inhibiting the generation of abnormal scars post injury. Finally, the microneedle patch was applied on the caudal region of a hypertrophic scar on a female patient’s dorsum to verify its clinic efficacy. Without any treatment, there was barely any dead keloid fibroblast in the 2D culture. After 12-hour treatment with the microneedle patch, the death rate increased to 83.8±11.96%. In rabbit ear hypertrophic scar model, 100% of the control wounds without the presence of patches on rabbit ears generated regions of raised dermis originating from the wound site (3/3), whereas microneedle treatment prevented dermis tissue thickening in 83.33% of the wounds (15/18). In the clinical test, the microneedle patch was well-tolerated by the patient. Compared to the untreated region, microneedle treatment decreased the quantity of infiltrated inflammatory cells, with less disrupted dermis tissue architecture and more flattened appearance.
Keywords: nanoparticle, microneedles, abnormal scar, keloid, hypertrophic scar
REFERENCE
- Qingqing Miao, David C. Yeo, Chenjie Xu*, Kanyi Pu*. Near-Infrared Fluorescent Molecular Probe for Sensitive Imaging of Keloid, Angewandte Chemie, DOI: 10.1002/anie.201710727
- Shiying Liu, David C Yeo, Christian Wiraja, HongLiang Tey, Milan Mrksich, Chenjie Xu*. Peptide delivery with poly(ethylene glycol) diacrylate microneedles through swelling effect, Bioengineering & Translational Medicine, 2017, DOI: 10.1002/btm2.10070
- David Yeo, Elizabeth Balmayor, Jan-Thorsten Schantz, Chenjie Xu*. “Microneedle Physical Contact as a Therapeutic for Abnormal Scars”. European Journal of Medical Research, 2017, 22(1):28
- Xue P, Yeo DCL, Chuah YJ, Kang YJ, Xu CJ*. Drug-eluting Microneedles for the Self-management of Keloids. Technology. 2014; 2(2): 144-152
Role of Mechanosignaling Pathways in Keloid Formation and Progression
Hesham Moneer Ahmad, MD
Associate professor, Dermatology Department,
Gulf Medical University, United Arab Emirates
Assistant professor, Dermatology Department,
Minia University, Egypt
Keloids are fibroproliferative skin disorders that are characterized by the accumulation of fibroblasts and collagens. Although increasing lines of research on keloids have revealed genetic and environmental factors that contribute to their formation, the exact etiology of these scars remains unclear. Recently it was found that increased mechanical stresses in the wound environment induce hypertrophic scarring via activation of mechanotransduction pathways. Mechanotransduction is the process by which physical forces are converted into biochemical signals that are then integrated into cellular responses. Mechanical stimulation modulates integrin, Wingless-type, protein kinase B, and focal adhesion kinase, resulting in cell proliferation and, ultimately, fibrosis. The discovery and development of various molecular pathways involved in this process have revolutionized the fundamental and clinical understanding regarding the formation and progression of cutaneous scars. Furthermore, mechanotransduction pathways are potential targets to reduce excessive scar formation. The aim of this presentation is to address the recent advances in scar mechanosignaling research.
Vision / Future of Keloid Research
Early Inflammatory Differences Between Normotrophic and Hypertrophic Scars in Humans
Frank B. Niessen, MD, PhD1;
Professor Susan Gibbs2
Introduction: Hypertrophic scar formation is a result of adverse cutaneous wound healing. The pathogenesis of hypertrophic scar formation is still poorly understood. A problem next to the lack of suitable animal models, is that most studies compare normal skin to hypertrophic scar samples and rarely to normotrophic scar samples. Also often only one time point after wounding is studied.
Material and Methods: In this study we performed a microarray analysis on material of human normotrophic (n = 6) and hypertrophic pre-sternal scars (n = 5) at 6 different time points (before wounding and up to 52 weeks after wounding).
Results: RNA levels for several factors involved in different phases of cutaneous wound healing, especially in the inflammation and proliferation phase (e.g. IL- 1α, CCL2, bFGF) were decreased in hypertrophic scars compared to normotrophic scars. Gene levels were only increased in hypertrophic scar compared to normotrophic scars for factors involved in matrix production, remodelling or degradation (Col3A1, THBS1, PLAU, TGFb3). The RNA data was confirmed by immunofluorescence protein staining of tissue sections for several of the biomarkers: CCL2, TGF-b3 and bFGF.
Conclusions: Generally it is described that hypertrophic scar formation is observed after an exaggerated inflammation. In contrast, our data suggests that hypertrophic scar formation is related to an extended down regulation of inflammatory and mitogenic genes whereas genes involved in matrix production remain up regulated thus resulting in an overproduction of extracellular matrix. This study has enabled us to identify typical biomarkers characteristic for hypertrophic scar formation.
Embryonic Stem Cell-Like Population within KALTS in Keloid Disorder Expresses Components of the Renin-angiotensin System and Cathepsins B, D and G
Swee T Tan MBBS FRACS PhD1,2, swee.tan@gmri.
org.nz; Hugo Humphries1, humphrieshn@gmail. com; Claudia Paterson1, claudiapatersonnz@gmail. com; Valerie M Y Lee1, minyi.lee@windowslive.com; Chelsea Grant BBiomedSc1, cch.grant@gmail.com; Helen D Brasch MBChB FRACP1, helen.brasch@ gmri.org.nz; Bede van Schaijik B Tech1 (Hons), bede.vanschaijik@gmri.org.nz; Jennifer de Jongh BBiomedSc1, jennifer.dejongh@hotmail.com; Paul F Davis PhD1, paul.davis@gmri.org.nz; Tinte Itinteang MBBS PhD1 tinte.itinteang@gmri.org.nz
ABSTRACT
Background: We have recently demonstrated the expression of embryonic stem cell (ESC)-associated markers OCT4, SOX2, pSTAT3 and NANOG by the endothelium of the microvessels within keloidassociated lymphoid tissues (KALTs) which constitute an aggregation of organised lymphocytes surrounding the microvessels. These findings show a unique niche of primitive cells within keloid disorder (KD) expressing ESC markers, revealing a potential therapeutic target for the treatment of this enigmatic condition. The renin-angiotensin system (RAS) plays a key role in stem cell proliferation and its dysregulation may lead to aberrant proliferation of fibroblasts within keloid lesions (KL). The RAS can be inhibited by commonly available medications, although enzymes such as cathepsins B, D and G may act as bypass loops for the RAS, hence reducing the effectiveness of the RAS inhibitors. This study investigated the expression and localisation of components of the RAS: pro-renin receptor (PRR), angiotensin converting enzyme (ACE), angiotensin II receptor 1 (ATIIR1), angiotensin II receptor 2 (ATIIR2), and cathepsins B, D and G, in relation to the putative ESC-like population on the endothelium of microvessels within the KALTs.
Methods: 3,3 Diaminobenzidine (DAB) immunohistochemical (IHC) staining for PRR, ACE, ATIIR1 and ATIIR2, and cathepsins B, D and G, was performed on 4μm-thick formalin-fixed paraffin-embedded sections of KL tissue samples from 4 female and 6 male patients, with a mean age of 28 (range, 7-55) years. Immunofluorescence (IF) IHC staining of 3 of these KL tissue samples was performed to localise the expression of PRR, ACE, ATIIR1 and ATIIR2 by co-staining with CD34, ERG, OCT4 or tryptase; and of cathepsins B, D and G by co-staining with CD34, OCT4 or tryptase. Western blotting (WB) and RT-qPCR were performed on 5 snap-frozen KL tissue samples and 4 primary cell lines derived from these KL tissue samples, to investigate protein and mRNA expression of these components of the RAS and the cathespins, respectively. Enzyme activity assays were performed on 5 snap-frozen KL tissue samples of the original cohort of 10 patients, to investigate the functional activity of the cathepsins.
Results: DAB IHC staining demonstrated expression of PRR, ACE, ATIIR1, ATIIR2 and cathepsins B, D and G in all 10 KL tissue samples. IF IHC staining demonstrated localisation of PRR, ATIIR2, cathepsins B and D to the OCT4+ endothelium of the microvessels, while cathepsin G was localised to the tryptase+ cells, within the KALTs. WB and RT-qPCR confirmed the presence of PRR, ACE, ATIIR1 and cathepsins B and D in the Kl tissue samples and the KL-derived primary cell lines. ATIIR2 and cathepsin G were only detected in KL tissue samples. Enzyme activity assays demonstrated functional activity of cathepsins B and D within the KL tissue samples.
Conclusion: PRR, ACE, ATIIR1, ATIIR2 and cathepsins B, D and G were expressed by the ESC-like population within the KALTs in KD. Cathepsins B, D and G may act as bypass loops for the RAS. These novel findings suggest the ESC-like population within the KALTs may be a potential therapeutic target using drugs that inhibit the RAS and its bypass loops.
Contact Information
Dr Swee Tan MBBS FRACS PhD
Gillies McIndoe Research Institute
PO Box 7184, Newtown, Wellington 6242,
New Zealand
swee.tan@gmri.org.nz
Funding source: This abstract was not funded by a government, pharmaceutical or biotechnology company, a foundation or another source.
Identification of Original Research: This is an original research
IRB / Ethics Committee Approval This study was approved by the Central Health and Disabilities Ethics Committee (ref. no. 13/NTB/155). Written consent was obtained from all patients.
Disclosure Declaration: Drs Tinte Itinteang, Paul Davis and Swee Tan are inventors of a patent application Treatment of Fibrotic Conditions (PCT/ NZ2016/050187). The authors are otherwise not aware of any commercial associations or financial relationships that might pose or create a conflict of interest with information presented in this abstract.
Evolution of Management of Keloids-From What it Was , What it is and the Things to Come in Future
Kamal Malaker, MD
De-activating Activin: A New Direction in the Treatment of Keloid Lesions
Peter Temple-Smith PhD 1,3*, Seungmin Ham1,3*, Craig Harrison2, Euan Wallace1,3 and Graeme Southwick1,4
Wound repair is a complex process involving various cytokines from the TGF-β superfamily including activins which are upregulated during wound repair with resulting fibrosis and scar. Our laboratory has recently shown that activin A gene and protein expression is significantly upregulated in keloid fibroblasts by the autocrine actions of activin when compared to normal fibroblasts. However, as no effective treatments for keloid disorder are currently available, we examine the use of follistatin, which binds to and neutralizes activins, as a potential treatment for fibrotic actions of dermal fibroblasts in vitro.
Normal and keloid fibroblasts were isolated and cultured in vitro using standard fibroblast cell culture protocols and their relative gene expressions were examined using qRT-PCR and RNAseq. Protein levels of activin and follistatin were also measured by enzymelinked immunosorbent assay and radioimmunoassay respectively. Cells were also treated with 100ng/ml follistatin 288—the more potent and locally-acting isoform of follistatin—for 5 days to examine the effects on the expression of fibrosis-related genes.
Keloid fibroblasts displayed elevated levels of activin A gene and protein expression through an activin autocrine pathway. These activin effects were gradually stimulated during in vitro cell culture. After a single treatment with follistatin 288, activin A gene expression in keloid fibroblasts was significantly reduced to normal fibroblast levels confirming that the autocrine actions of activins in keloid fibroblasts are inhibited by this treatment. Moreover, downstream targets of activins such as connective tissue growth factor (CTGF) declined significantly in keloid fibroblasts compared to normal controls.
Keloid disorder and the development of keloid lesions is linked to the local production of activin A. The action of follistatin in suppressing activin A and CTGF gene expression suggests a novel role for this protein in the treatment of keloid and other fibrotic disorders.
Changes to Fibroblast Phenotype in Keloid Disorder
Andrew Stevenson1, Zhenjun Deng1, Manon Subilia1, Mansour Alghamdi2, Nutan Chaudhari1, Nicole Hortin1, Helen Douglas,1,3, Suzanne Rea1,3, Fiona Wood 1,3 and Mark Fear1
Keloid disorder is a fibroproliferative disorder of the skin of unknown pathophysiology. Whilst the pathophysiology is not yet defined it is well known that excessive and progressive deposition of extracellular matrix (ECM) and in particular Collagen is a hallmark of this fibrotic condition. The key cell producing this matrix is the fibroblast, which plays a major role particularly in wound repair, scarring and fibrotic disease. However, despite their importance, fibroblasts remain poorly characterized and the level of heterogeneity within tissue populations ill-defined. Recent studies have revealed the presence of distinct lineages of dermal fibroblasts with different roles in fibrosis using mouse models. However, the existence and importance of fibroblast subtypes in normal skin and fibrosis in humans is not known.
We have investigated changes to fibroblast phenotype and the heterogeneity of fibroblast phenotype in skin and keloid disorder. We have focused on changes in the transcriptome, matrix production and response to extracellular matrix biological and physical properties to understand how normal fibroblast function is disrupted in keloid disease at the molecular and cellular level. Keloid tissue appears to contain multiple distinct subsets of fibroblasts with differential activity, in particular with respect to matrix production. In addition, cellmatrix interactions and responses also appear to be disrupted in keloid derived fibroblasts. Together the data suggests changes in fibroblast heterogeneity and their response to ECM may be important in keloid disease. Further investigation and characterization of specific fibroblast sub-types may facilitate a better understanding of the disease and ultimately better targeted interventions to ameliorate fibrosis.
Shortcomings of Surgery in Treatment of Keloids / Debate / Clinical Case Presentations
Michael H Tirgan MD
Clinical Research Session / Abstract Presentation
Incidence of Hypertension among Keloid Patients
Joel Correa da Rosa, PhD
Efficacy of a Multimodal Approach for treatment of Keloids
J. Gerardo Garcia, MD, A. Kate Arefnia, MD and Reza F Ghohestani, MD, PhD Texas Institute of Dermatology, San Antonio, TX 78257, USA. director@txid.org
Goal: Purpose of the study is to establish a two-step treatment approach for treatment of keloids since any single therapy system has failed to provide a high success rate.
Methods & Results: A total of 257 patients were treated by different modalities including Intralesional steroid injections, Cryotherapy with liquid nitrogen, light therapy, Fractional CO2 laser, or Surgical excision. Our data clearly shows that excision should be reserved for very rare cases due to a very high recurrence rate.
Conclusion: A multimodal approach consists of intralesional Kenalog and Cryotherapy is the most effective method for successful treatment of keloids.
Vitamin D Receptor Expression in Keloids
Dorothy M. Supp, PhD
Shriners Hospitals for Children-Cincinnati, Research
Department, and The University of Cincinnati
College of Medicine, Department of Surgery,
Cincinnati, Ohio, USA
Keloids are abnormal fibroproliferative scars that pose a significant challenge to patients and clinicians. The molecular basis for keloid formation remains incompletely understood, and currently no universally effective treatments exist. It is well recognized that keloids are more prevalent in populations with darkly pigmented skin, such as African Americans, but the basis for the link between skin color and keloid risk is not known. Dark skin pigmentation is due to high levels of melanin, which shields keratinocytes from ultraviolet (UV) light. Although UV exposure is a known risk factor for skin cancer development, it also has beneficial effects; notably, epidermal keratinocytes synthesize the active form of vitamin D upon exposure to UV light. Vitamin D produced in the skin is considered to be the major source of vitamin D in humans. African Americans have a greater incidence of vitamin D insufficiency compared to non-Hispanic whites, which was proposed to result in part from reduced vitamin D production in darkly pigmented skin due to high levels of melanin. Hypothetically, reduced vitamin D levels in individuals with darkly pigmented skin may contribute to the risk of keloid scar formation. In addition to regulation of calcium homeostasis, vitamin D plays important roles in cell proliferation, differentiation, cancer progression, inflammation, and fibrosis. The activities of vitamin D are dependent on the vitamin D receptor (VDR), a member of the steroid nuclear receptor superfamily. The ligand-bound VDR acts as a transcription factor; thus, nuclear localization is required for liganddependent regulation of target gene expression. We investigated expression and nuclear localization of VDR in biopsies of keloid scars and normal human skin (N = 24/group). VDR protein levels were reduced in a majority of keloid scars. Further, the percentage of epidermal cells displaying nuclear VDR localization was significantly lower in keloid scars compared with normal skin samples. Interestingly, analysis of VDRpositive nuclei among different normal skin samples showed a significant reduction in nuclear localization in epidermis of black donors compared with white donors; a similar trend was observed in keloid scar samples, but the difference was not statistically significant due to the relatively small number of keloid scars obtained from white patients. To determine if keloid cells are competent to respond to vitamin D stimulation, we studied keratinocytes isolated from keloid scars and normal skin samples (N = 4/group). VDR mRNA expression levels were similar in keloid and normal keratinocytes in vitro, and stimulation with vitamin D significantly increased expression of the vitamin D target genes Cathelicidin and CYP24A1 in both groups of cells. The results suggest that VDR is involved in keloid pathology and hint at a possible role for VDR in the increased susceptibility to keloid scarring in individuals with darkly pigmented skin. Further, because keloid keratinocytes can respond to vitamin D treatment by appropriate upregulation of target genes, the results suggest a potential therapeutic role for vitamin D in prevention or treatment of keloids.
Study of Hyperbaric Oxygen Treatment in Keloid Patients
Wang Youbin, MD
Plastic Department of Peking Union Medical College
Hospital, Peking, China. wybenz@sina.com
Keloids are fibrous growths that extend beyond the original area of injury to involve the adjacent normal skin. In the last few decades, Hyperbaric oxygen (HBO) therapy has been introduce to treat multiple injuries and disorders. HBO can improve the level of dissolved oxygen and oxygen diffusion capacity in blood, it can also enhance the immune system and microcirculation function. By reducing the role of capillary permeability , HBO can also improve edema effectively. However, the effect of HBO on prevention of keloid is not quite clear in the past. Through various research, we think the answer is “YES”.
In our study, HBO can reduce the post-surgery recurrence rate on patients diagnosed with keloid significantly. HBO can also alleviate the pruritus suffered by keloid patients, which is a main complaint of them in the outpatient. All these points we said is about to effect the keloid tissue, however, we found that HBO can even obstruct the formation of keloid by influence the epithelial-mesenchymal transition (EMT), which is quite meaningful to our clinic works.
To sum up, Hyperbaric oxygen has a solid molecular biological basis for the prevention and treatment of keloid. In addition, the effect of HBO on curing keloid is preliminary supported in our daily clinical works.
In the future, we will continue focusing on the application of HBO to prevent and retard the progress of keloid formation. Hopefully, we can combine HBO and other non-surgical method, which will be good news for the vast patients suffered from keloid.
Multiscale Analysis of A Keloid by
Imaging and Biomechanical Devices
Chambert J.1,2, Lihoreau T.3, Joly S.1,2, Chatelain B.4, Jacquet E.1,2 and Rolin G.3,5
ABSTRACT
Keloid scars are benign skin tumors-like that consist in the excessive accumulation of fibrotic tissue that extends beyond the original wound [1]. Keloids are located on specific sites, tend to over grow during time, and cause cosmetic and psychological issues to patients. The physiopathology of keloids remains unclear and there is still no therapeutic consensus. Nevertheless, it is now agreed that keloids result from a combination of genetic, cellular and mechanical factors [2].
The present study deals with a multi-devices experimental analysis and aimed to fully characterize the mechanical behavior of a keloid compared to its surrounding cutaneaous tissue and the contralateral healthy skin. A keloid (butterfly-shaped), localised on the upper-left arm of a female volonteer was analyzed non-invasively in accordance with the Declaration of Helsinki.
Mechanical investigations were coupled with imaging measurement (OCT, confocal in vivo and echograph) in order to adress the complex tissue heterogeneity of keloid tissue. Suction tests have been carried out with a SEM 575 Cutometer® (Courage & Khazaka Electronic GmbH, Cologne, Germany). A measuring probe was placed on skin and a negative pression of 400 mbar was applied during 3 seconds (cycle of loading, holding time and unloading) followed by a 2-seconds relaxation time. This process was repeated three times to determine tissue elasticity. Then, the ultra-light uniaxial mechanical device developped by Jacquet et al. [3] has been used to highlight the non-linear hyperelasticity and viscosity of keloid scar. Briefly, the device was equiped with a displacement and force sensors which allow a noninvasive in vivo traction test. Specific pads have been designed to reduce the influence of surrounding peripheral skin on measurments. Datas (displacement and force) were aquired during three successive loading-unloading sequences at a constant strain rate. Thus, tissue thicknesses have been measured by 20 MHz Atys Dermcup® Ultrasound echograph to determine the stress-strain curve of each tissue. Then, displacement fields on the surface of each tissue were obtained by a digital image correlation method.
Mechanical tests have been first performed on two healthy symetrical anatomic sites (forearms) and allowed the validation of the hypothesis that symetrical anatomic sites have similar mechanical behavior. From the extension test, keloid tissue was shown to be strongly stiffer than healthy skin and the keloid extensibility was about two times less than healthy one. Typical R-parameters calculated from cutometry have been identified and provide the elasticity, viscoelasticity and fatigability of skin [4]. The obtained results have shown a decrease of elastic properties of keloid compared to the healthy skin ones. In parallel, all these keloid features were confirmed and quantified by displacement fields.
As a conclusion, mechanical properties of keloid, compared to surrounding and controlatral healthy skin, have not only been identified and compared here but were also correlated through a multiscale analysis. Further biological studies should elucidate the mechanisms underlying keloids in order to propose more effective prophylaxis and treatment strategies [5]. In a biomechanical and complementary way, our multiscale approach could let reseachers and clinicians rely on quantitative reference data. These data could be used to monitor the evolution of keloid scars, to anticipate keloid recurrence or to assess the anti-fibrotic effect of a treatment.
Contact information
Dr. Gwenaël ROLIN, PhD
Hospital Research Engineer
INSERM CIC-1431, University Hospital of
Besançon, Clinical Investigation Center
2 place St Jacques – 25000 Besançon
grolin@chu-besancon.fr
Office: +33 (0)3 81 21 91 64
Mobile: +33(0)6 84 25 77 92
Funding source
This work was funding by the “European Union” and “Région Bourgogne Franche-Comté” (Grant36381).
REFERENCES
- Philandrianos C, Kerfant N, Jaloux C Jr, Martinet L, Bertrand B and Casanova D. Keloid scars (part I): Clinical presentation, epidemiology, histology and pathogenesis. Ann Chir Plast Esthet. 2016. 61:128-35.
- Harn HI, Ogawa R, Hsu CK, Hughes MW, Tang MJ and Chuong CM. The tension biology of wound healing. Exp Dermatol. 2017. 4. doi: 10.1111/exd.13460. [Epub ahead of print]
- Jacquet, E., Joly, S., Chambert, J., Rekik, K., and Sandoz, P. (2017). Ultra-light extensometer for the assessment of the mechanical properties of the human skin in-vivo. Skin Research and Technology, 491-499.
- Dobrev, H. (2014). Cutometer . In: Berardesca, E., Maibach, H.I., and Wilhelm, K.P. (Eds.), Non Invasive Diagnostic Techniques in Clinical Dermatology. Springer, Ch. 29, 315-338.
- Lee HJ and Jang YJ. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int J Mol Sci. 2018. 19, 711.
CLINICAL ABSTRACT PRESENTATION: Use of Pneumatic Needle-Free Injection Technology for Treatment of Keloid Scars in Adult Patients
Alex Levenberg, MD; Daniel Cassuto, MD
ABSTRACT
Hypothesis /Aims Of Study: Currently recommended treatment regimens include intralesional injection of 5-FU combined with corticosteroids in several ratios and proportions. The purpose is to attain stops cell proliferation in the scar tissue with the cytotoxic antimetabolite and achieve a high local concentration of the corticosteroid at the diseased site without significant systemic absorption. Side effects include injection pain, thinning and atrophy of the skin and subcutaneous tissues, capillary dilation, and development of secondary hypopigmentation. Needle-free injection technology of EnerJet designed to introduce and laterally disperse a therapeutic substance into the dermis via pneumatic needle-less action. The depth of penetration (ranging from 1-5 mm depth) is system-controlled and the administered substance uniformly distributed to approximately 1cm2 area intra-dermally threedimensionally around every injection point. The current pilot clinical trial evaluates the efficacy of intralesional delivery of methylprednisolone acetate and 5-FU with the EnerJet system in improving appearance and symptoms associated with keloid scars of various etiologies in adult patients.
Study Design, Materials and Methods: This is an ongoing physician-initiated open label study in which feasibility, tolerability, efficacy and safety of intralesional administration of DM and 5-FU are evaluated. Patients are considered eligible if they were diagnosed with keloid lesions ≥ 1 cm in diameter located in torso or upper arm or forearm areas with estimated scar age ≤5 years. Subjects with hypertrophic scarring, history of vascular or bleeding disorders, diabetes mellitus, renal, hepatic or respiratory failure, documented anemia, leukopenia, thrombocytopenia, infection, bone marrow depletion, known hypersensitivity to corticosteroids or lidocaine, chronic use of systemic corticosteroids or immunosuppressive medication, previous treatment with intralesional steroids within 6 months, and pregnant or lactating females were excluded. Twelve patients (male n=5, female n=7, age range 17-74) were enrolled in the trial and received multiple DM+5-FU injections. Enrolled patients underwent multiple bi-weekly intralesional injections performed in 1-cm grid over the keloid and its borderline. Subjects are evaluated at screening and 3-month after the last treatment session (follow-up, FU). The safety and tolerability endpoints include AEs and injection pain (VAS). The efficacy outcome measures taken at baseline and FU include VSS, POSAS, and assessment of the photo images taken at baseline and at FU. Final complete results are planned to be available in may, in time for the meeting.
Results: No patient discontinued the trial. Currently, 4 patients completed intervention period and are awaiting FU assessment. At baseline, patients complained of keloid itching and pain, had significant lesions (VSS range 5-10) with severe burden (POSAS range 17-55). Currently, no AEs occurred. The average injection VAS score is 3 and indicated mild pain. As of now, the entire treated and undergoing treatment patients reported complete disappearance of the associated symptoms. All lesions show changes in the appearance: flattening of the lesions, softening and normalization of the color.
Interpretation of Results: Current results suggest that intralesional injection of DM+5-FU with EnerJet system is generally safe and well tolerated. No AEs were reported. In contrast to intralesional needle injections, no discomfort or pain associated with the procedure was observed and it was well tolerated. The apparent positive improvement in keloid lesions and drastic decrease in pain and itching are encouraging and provide the basis for further exploration of the safety and efficacy of DM+5-FU in placebo controlled trial.
Concluding Message: As this form of treatment has the potential for being a viable treatment option, further exploration of the efficacy and safety of DM mixed with 5-FU is warranted.
Special Sessions
RANDOMIZED STUDY OF EAR KELOIDS RCT: Contact Cryosurgery vs Surgical Ablation Followed by 5-Fluorouracil to Treat Ear Keloids
Jürg Hafner, Zürich, Switzerland
INTRODUCTION
Both contact cryosurgery and CO2-laser ablation plus 5-fluloruracil (5FU) are effective treatments for ear keloids. Rates of complete or partial remission, and recurrence, respectively, are unknown.
STUDY DESIGN
Randomized controlled study on contact cryosurgery versus surgical ablation followed by 5FU
METHODS
Inclusion: Patients with ear keloids at any location of the auricle or lobe, > 0.5 cm large.
Exclusion: Pregnancy, sensitization to 5FU, medication with brivudine or capecitabine, phobia of syringes.
Study duration: 2 years per included patient Primary end point: Complete remission.
Secondary end points: Partial remission, recurrence. Subjective improvement (esthetics, hypersensitivity, pruritus)
Schedule: Initial treatment. Monthly control with optional retreatment: Cryosurgery patients may have repeated cryosurgery every 4 weeks as indicated. Surgically treated patients may have 5FU infiltrations every 4 weeks as indicated. Continued 4-weekly follow-up for 2 years.
Statistics: Sample size: n=161 (power 80%): Assumption (1): treatment A is 80% and treatment B is 60% effective; assumption (2): total drop out rate of 30% in 2 years of follow-up. Randomization in blocks of 8. Multicenter trial.
Bilateral Annular Breast Keloids that Developed as a Paraneoplastic Phenomenon and Showed Unique Immunohistochemical Findings in an Elderly Woman Associated with Bilateral Breast Cancers
Masahiro Oka, MD, PhD1; Takeshi Fukumoto, MD, PhD2; Fumiyoshi Fujishima, MD, PhD3; Kaori Fukuda, MD4; Masao Ban, MD5; Takeshi Kozaru, MD1
ABSTRACT
We report a case of bilateral annular breast keloids that developed as a paraneoplastic phenomenon and showed unique immunohistochemical findings in a 72-year-old woman associated with bilateral breast cancers. The patient presented with a 2-month history of itchy skin lesions on both breasts. The lesions had been gradually extending peripherally. Three years earlier, she had been diagnosed with bilateral breast cancers associated with multiple bone metastases. Physical examination revealed an annular, reddishbrown plaque with a slightly elevated, expanding border on the lower surface of the right breast. The central portion of the plaque showed healing tendencies and the overlying skin seemed normal. On the left breast, a reddish-brown plaque showing central healing tendency was present on the lower surface. Based on clinical and histopathological findings, the breast skin lesions were diagnosed as keloids. The keloids showed two interesting immunohistochemical findings. First, they showed unique distribution of a-smooth muscle actin (SMA)+, CD34- myofibroblasts and a-SMA-, CD34+ fibroblasts depending on the region. Specifically, the polygonal cells in the elevated portion were positive for a-SMA and vimentin, but negative for CD34, indicating that these cells were myofibroblasts. On the other hand, spindle cells in the central portion of keloid were completely negative for a-SMA, but positive for vimentin and CD34 indicating that these cells were fibroblasts. These results not only indicated an inverse relationship between CD34 expression and myofibroblastic differentiation in fibroblasts, but also revealed a unique distribution of fibroblasts and myofibroblasts in keloid growing peripherally. Second, CD163-positive M2 macrophages, which can produce tumor growth factor (TGF)-b, were abundantly detected in the elevated portion of keloid, while these cells were considerably less numerous in the central healing portion compared to the elevated expanding portion. One interesting idea based on these results is that high levels of TGF-b released from CD163- positive cells played a crucial role in the formation of breast keloids through active induction of fibroblast differentiation into myofibroblasts. The present case strongly supports the previously proposed idea that keloid can form as a paraneoplastic phenomenon in breast cancer patients.
Contact information
Masahiro Oka, MD, PhD
Division of Dermatology, Tohoku Medical and
Pharmaceutical University Sendai 983-8536, Japan
Tel: +81-22-352-1227
Fax: +81-22-259-1232
e-mail: oka@hosp.tohoku-mpu.ac.jp
Laser Treatment of Keloid Lesions, Efficacy and Side Effects, Results of an on-line survey.
Michael H. Tirgan, MD
INTRODUCTION
Lasers are among the most commonly used tools in day to day practice of dermatology. Although there are several reports about the utility and efficacy of laser treatment in keloid lesions, there is a paucity of literature as to how keloid patients perceive the efficacy of this modality.
MATERIAL AND METHODS
An online keloid survey was launched in November 2011. Participants were asked to provide answers to numerous questions about their keloid disorder, including their perception of the efficacy of laser for treatment of their keloidal lesions. Descriptive statistics are provided.
RESULTS
As of November 20, 2017, total of 1597 individuals participated in this survey. 191 participants indicated that they had previously received at least one laser treatment for their keloids, among whom 174 provided an assessment of the benefit of this intervention. Five patients (2.9%) reported that laser treatment cured their keloids. 47 patients (27%) reported having benefited from the treatment. 82 patients (47.1%) reported no improvements, but most interestingly, 40 patients (23%) reported that laser treatment caused worsening of their keloids.
DISCUSSION
With several limitations, this study represents the first step in developing a patient-reported measure of treatment success and benefit drawn from laser treatment. The most important finding of this study is that 23% of patients reported worsening of their keloids with this treatment. Worsening of keloids after laser treatments has never been reported.
Post Otoplasty Keloids
Michael H. Tirgan, MD
INTRODUCTION
Safe and effective treatments are needed for otoplasty induced posterior auricular keloids. Objective of this study was to review clinical presentation and natural history of post-otoplasty keloids, and to assess impact of non-surgical therapeutic intervention with cryotherapy as primary treatment in order to reduce the rate of recurrence as well as worsening of keloids after surgery.
MATERIAL AND METHODS
14 patients with posterior auricular keloids were seen by author in his New York City keloid specialty medical practice. Medical records and photographs of the lesions were reviewed in a retrospective manner. Cryotherapy as the main and primary treatment was offered to 12 patients with bulky keloid lesions.
RESULTS
Among 14 patients (23 ears with keloids), two patients (four ears) had minimal keloid formation at the site of surgery and were advised to primarily receive, or continue with, intra-lesional steroids. Twelve patients had bulky keloids and were offered cryotherapy. Eleven patients consented to treatment. As of the date of this publication, seven patients have achieved desirable results and are happy with their outcome. Four patients are still receiving treatment.
DISCUSSION
Most patients with post otoplasty keloids receive variety of treatments over an extended period of time. Common treatments include surgery, intra-lesionals steroids and radiation therapy. Keloid is a genetic disorder of wound healing mechanism, triggered by injury to the skin. Attempting to remove post otoplasty keloids with surgery naturally results in wounding of the skin and a pathological healing response that often leads to formation of a much larger keloid. Radiation therapy is a known carcinogen, especially when used in young patients.
Cryotherapy seems not to trigger wound healing response to the extent that surgical scalpels do, therefore, it can result in a much better therapeutic outcome. Using cryotherapy as primary intervention for bulky posterior auricular keloids can successfully reduce the bulk of these lesions and not cause worsening of keloids. Additionally, cryotherapy is less morbid, less costly, and very easy to administer in outpatient setting.
Intralesional Triamcinolone Acetonide in the Treatment of Keloid Lesions: Can the treatment be harmful to some patients? Results of an online survey.
Lennert Van Putte, MD; Michael H. Tirgan, MD
INTRODUCTION
Intra-lesional Steroid (ILS) Injection is the most commonly used treatment modality for Keloid Disorder (KD). An IRB approved online survey was launched in November 2011. Patients’ perception of the efficacy of ILS in KD is reported here with special attention to worsening of keloids in 16.6% of cases.
MATERIAL AND METHODS
As of September 23, 2016, 1406 consecutive unselected patients participated in this survey. 777 patients reported having had ILS injections for KD and we able to provide an assessment of the efficacy of ILS on their KD. Updated Dataset will be presented at the meeting.
RESULTS
Only 0 patients (1.2%) reported that ILS cured their keloids. ILS improved KD in 254 patients (32.7%). 385 patients (49.5%) reported no improvement with ILS and an additional 129 patients (16.6%) reported ILS causing worsening of their KD.
DISCUSSION
With several limitations, this study suggests that only one third of patients have Steroid Sensitive KD and two thirds have Steroid Resistant KD. The most interesting finding of this study is that 129 patients (16.6%) reported worsening of their keloids after ILS injections. Worsening of keloids after ILS has not been reported previously. Keloids occur as a result of dermal injury in genetically susceptible individuals. ILS-induced tissue injury in Steroid Resistant KD can lead to formation of a new keloid or aggravation of a previously formed keloid.
Massive ear keloids: Natural history, evaluation of risk factors and recommendation for preventive measures – A retrospective case series of 283 patients with ear keloids.
Michael H. Tirgan, MD
INTRODUCTION
Keloid Disorder (KD) is an inherited wound healing ailment, frequently seen among Africans /African Americans and Asians. Ears are common locations for development of keloids. This review focuses on natural history of massive ear keloids and risk factors that lead to formation of these life-changing and debilitating tumors and recommendations for prevention.
MATERIALS AND METHODS
This is a retrospective analysis of 283 consecutive patients with ear keloids. Keloids were assessed visually and categorized according to their size.
- Massive ear keloids, whereby the size of the keloid mass was greater than the surface area of the corresponding ear. Thirteen patients (4.5%) met this criterion.
- Semi-massive ear keloids, whereby the size keloid mass was at least 50 % of the surface area of the corresponding ear. Eighteen patients (6.3%) met this criterion.
- Large ear keloids, whereby the size of the keloid mass was more than the size of the corresponding earlobe. 181 patients met this criterion and were placed in this category.
Small ear keloids, whereby the size of the keloid mass was less than the size of the corresponding earlobe. Seventy-one patients met this criterion.
RESULTS
Despite the fact that this study is limited by its size and patients from only one medical practice that does not offer surgery for treatment of keloids, several interesting factors stand out as risk factors for development of large, semi-massive and massive ear keloids.
- Race stands out as a major potential risk factor in all four groups, most importantly among those with massive, semi-massive ear keloids, with only five Caucasians among 31 patients in both these groups.
- Prior keloid removal surgery was the most important risk factor among all 31 patients with massive and semi-massive ear keloids in this study. Without an exception, all patients had undergone anywhere between one to seven prior keloid removal surgeries.
- Prior keloid removal surgery was the most important risk factor, among all 181 patients with large ear keloids in this study with 131 patients (73%) having had history of prior keloid removal surgery. History of prior keloid removal surgery is summarized in table 3.
DISCUSSION
Surgical removal of keloids will indeed trigger this pathological wound healing response and therefore, can result in development of a much larger ear keloid. In the author’s opinion, the paradigm shifting approach is a move to utilize cryotherapy for treatment of all primary and secondary ear keloids.
Keloid Surgery and Adjuvant Therapy Innovation: Ten Years Experiences and 1500 Cases Analysis
Wang Youbin
Plastic Department of Peking Union Medical College
Hospital, Peking, China.
wybenz@sina.com
Keloid is defined as excessive scar tissue formation and usually occurs as a result of pathological wound healing after trauma and inflammation. It may be induced by acne, foliculitis, surgery or other skin injuries. Many parts of the body are involved, especially chest wall, shoulder and mandible. The reported therapies include surgical excision, irradiation, steroid injection and other methods. Surgical wound closure methods are very important in recurrence prevention. Meanwhile, cosmetic result is another indispensable issue to be considered in keloid treatment. Based on the principle of recurrence prevention and cosmetic consideration, we used double layer continuous intradermal suture besides basic surgery techniques in keloid operation. Meanwhile, many other surgical innovations have been proposed. Internal thoracic perforator vessel skin flap was used in front chest keloid which the wound can’t be closed directly after keloid excision. For large keloid with skin graft, pre-cut and pre-radiotherapy was used to reduce recurrence rate. Abdomen skin flap transplantation with vessel anastomosis was also used in large front chest keloid treatment. With “X-shape skin flap design”, the keloid skin dermis was saved for auricle keloid reconstruction. In our study, with 1800cGy post-surgical radiotherapy, 91.7% effective rate was reached in average one year post-operative following-up. According to the follow-up review of the therapeutic effect of 1500 cases of keloid patients, we have achieved lower recurrence rate and better clinical cosmetic results in the treatment of keloids through the improvement of the innovated surgical techniques and assistance with effective radiation therapy.
Estimates of Radiation Risks Arising from the Treatment of Keloids by Radiotherapy
Dr. Henry Weatherburn (Head of Physics), PhD1,
Dr. John Glees (Consultant Clinical Oncologist)
MD, FRCR1and Prof Kamal Malaker (Director of
Clinical Oncology, CCEC) MD, PhD, FRCS2
INTRODUCTION:
Quantitative estimates of the risk of side effects arising from the treatment of keloids by radiotherapy are important. These are determined both for excised and unresected keloid treatment regimes, particularly the risk of skin cancer induction.
METHOD:
For excised Keloids a standard treatment is the delivery of a dose of 10Gy in a single treatment fraction using a 60kV (or sometimes higher kV) X-ray beam or, alternatively, an electron beam. For unresected Keloids, two regimens are often employed: either a total of 16Gy given in four quarterly fractions over a period of a year; or 37.5Gy given in five once weekly fractions [1,2].
As ionising radiation is employed in this treatment, deterministic effects arise, both as acute effects, such as erythema, etc. and late effects, such as pigment changes, etc. A risk of stochastic effects, primarily skin cancer, is also present and a quantitative estimate of this risk is derived from radiobiological risk calculations and compared with risks reported in reviews.
To estimate the risk of skin cancer, calculations are undertaken for the treatment regimes described above noting that, if two or three keloids are irradiated, the risk will then be double or tripled.
An irradiated area of 20 cm2 is assumed and it will also be assumed that the surrounding site is protected by the lead applicator, with the remainder of the body receiving only minimal leakage and scattered radiation. Time gaps between treatment fractions are also ignored, and , in the first instance, the effect of age.
In calculating the risk initially only the skin is taken into account and other underlying irradiated tissues are ignored. The calculation assumes a standard middle-aged adult [3].
RESULTS:
The total body skin area is assumed to be 2 m2 and, assuming that the radiation is relatively superficially absorbed and the area of the keloid and the surrounding planned treatment margin only being exposed, 20 cm2 of skin is thus exposed with 10 Gy.
Using Wr = 1 for x-rays and Wt = 0.01 for skin, we obtain an effective dose to the irradiated skin of 0.1 mSv. For a patient having unresected three unresected keloids treated each with a dose of 37.5 Gy this could rise to 1.13 mSv (with a radiation risk coefficient of 0.01%) and can be compared with the effective dose for annual background radiation which, for the UK is approx 2.6 mSv.
Extending this model to various organs, muscles, bones, and bone marrow (e.g. for radiotherapy of a heel spur employing 200 kV x-rays, dose 12 Gy, area 80 cm2), where an effective dose for skin has been estimated to be 2.9 mSv, and, including all organs, this increases to 8 – 9.5 mSv, i.e. by a factor of approx. three [4]. The risk also increases by a factor of three at age 25 compared with age 50 and for women is double that for men.
DISCUSSION & CONCLUSIONS
Overall the risk arising from the treatment of large areas of skin with sensitive underlying tissue, e.g. mammary tissue in the breast, may increase risk of cancer induction to 0.1% – 1% and may explain a reported case of potentially radiation induced breast cancer [5].
Induced cancers reported in reviews of keloid treatment are at magnitudes of below 1 in 1,000 (i.e. < 0.1%), or as low as 1 in 10,000 (i.e. < 0.01%), which show a general correspondence with the above values [1,6,7].
REFERENCES
- Treatment of Keloids by Surgical Excision and immediate Postoperative Single-fraction Radiotherapy. Ragoowansi, R et al., Plastic and Reconstruct Surgery. (2003); 111: 1853-1859
- Retrospective Analysis of Treatment of Unresectable Keloids with Primary Radiation Over 25 Years. Malaker, K et al., Clinical Oncology (2004); 16: 290 – 298
- Annals of the ICRP Publication 103. The 2007 Recommendations of the International Commission on Radiological Protection Editor J. VALENTIN, Elsevier (2017)
- Estimation of the carcinogenic risk of benign diseases from shoulder to heel. Jansen J, Boerse J, et al, Radiotherapy and Oncology (2005); 76: 270 – 277
- The risks of treating keloids with radiotherapy. Botwood, N, et al. British Journal of Radiology (1999); 72: 1222 – 1224
- Is radiation therapy for keloids acceptable? The risk of long term carcinogenesis. Ogawa R, Myashita T et al, Plast Reconstr. Sirg. (2009); 124:1196 – 1201
- Radiation therapy for the Adjunctive Treatment of Surgically Excised Keloids: a Review Cheragi, N, Cognetts A, and Goldberg D J, Clinc Aesthet Dermatolog (2017); 10 (8): 12-18
